| A Study of the Neurophysiological Mechanisms of Dreaming |
| M. Jouvet and D. Jouvet Electroenceph. Clin. Neurophysiol. 1963 Suppl. 24 |
Dream activity can be detected only by the short memory of the dream. thus, any effort to delimit the neural structures responsible for such a mysterious phenomenon may appear very risky. Nevertheless, some recent data obtained on humans and animals may contribute to make such an effort less hazardous. Indeed, Kleitman and his collaborators have demonstrated the periodical appearance of a peculiar stage of sleep with low voltage EEG and rapid eye movements. The subjects, aroused during this stagc, remembered having dreamt in a great number of cases (Dement and Kleitman 1957a, b). ln cats, besides the slow cortical activity which is observed during physiological sleep (Rheinberger and Jasper 1937), the existence of fast cortical activity accompanied by rapid eye movements was recognized by Dement (1958). To the latter author, the fast activity stage of sleep in the cat represented an active phase of sleep (activated sleep) intermediate between deep sleep characterized by slow waves and the state of wakefulless. The electrical activity of the cortex and of the subcortical regions and the variations of somato-vegetative activity are so characteristic of the fast cortical activity stage of sleep that they make possible the study of the neural structures responsible for it (Jouvet and Michel 1958; Jouvet et al 1959b, 1960;Jouvet 1961, 1962). And the very surprising similarity between this stage of sleep in man and cat makes possible the correlation between a structural analysis in cat and the subjective data obtained in man.
We shall thus begin to enumerate some experimental facts obtained in cats which permit us to delimit a ponto-limbic system responsible for the fast cortical activity phase during sleep (Rhombencephalic Phase of Sleep, or RPS). Secondly, we will outline the striking similarities between the RPS and a stage of sleep observed in normal humans or in patients with brain lesions similar to experimental lesions carried out in cats. Finally, we shall discuss the relationship between this phase of sleep and dream activity.
Part I : Experimental results obtained on cats
The rhombencephalic phase of sleep
Methods
Results were obtained from 69 chronic cats (15 normal cats, 11 totally or partially decorticate, 20 with total or partial brain stem section, 21 with partial coagulation of the brain stem and 2 which had undergone total ablation of the cerebellum). These animals were implanted with nickel-chrome multipolar subcortical electrodes and steel cortical electrodes and were all studied for more than I week. Decortication was carried out in one operation by suction after removal of the skull. An acrylic resin plastic roof was then inserted in place of the bone and the subcortical electrodes were thus kept at that level. The brain stem was sectioned by means of a stereotaxically oriented cutting blade, and electrolytic lesions were made by means of anodal direct current. In some cases, the entire part of the brain rostral to the mesencephalon was removed by aspiration. Poikilotherm, mesencephalic or pontile animals were kept warm and were always studied at normal temperatures between 37° and 39° C. The cats were placed in soundproof cages and EEG recordings were carried out daily during 6-12 h. Routinely, together with the EEG, the EMG was recorded by means of a bipolar electrode permanently implanted at the level of the neck muscles. The eye movements were also recorded by electrodes fixed on both sides of the eyeballs. The behavior of the animals (pupils, nictitating membranes, posture, etc.) was observed through a window. In all cases, the site of the electrodes and the topography of the lesions were checked histologically.
Part 1 : Experimental results obtained on cats
The rhombencephalic phase of sleep
Results
I. Two EEG patterns of physiological sleep in intact cats
Two different stages can be distinguished from the records ( Fig. 1):
A. Falling asleep and Slow Phase of Sleep (SPS): This stage is marked by the appearance of spindles followed by slow waves which invade the cortex, the diencephalon and then the mesencephalic reticular formation (RF). At the same time, high voltage (300-500, microV) spikes of short duration (40-50 msec) appear in the limbic system (hippocampus, mamillary bodies and septum) (Jouvet et al. 1959a). During this stage, the animal prepares for sleep. The head is bent while the EMG of the neck muscles discretely falls. The respiration as well as the heart rate are regular and slow down in comparison with rates in the waking state. As the invasion of the reticular formation by the slow waves proceeds, the threshold of arousal obtained by direct electrical stimulation of the RF increases 20-40 per cent.
Whatever subcortical structures may be responsible for the synchronization phenomena (diffuse thalamic system, or caudal region of the brain stem—see Batini et al. 1959b), one fact appears to be certain: the spindles and the slow waves which are observed during the first phase of sleep require the presence of the neocortex, since there is no subcortical spindle or slow wave in the decorticate animal, nor behind a section of the brain stem at the mesodiencepha]ic level. Thus, the telencephalic origin of the slow phase of sleep is probable. Such a "telencephalic sleep" would express the activity of some corticofugal mechanism which has been discussed elsewhere (Jouvet and Michel 1958; Jouvet 1962).
B. Fast phase of sleep or Rhombencephalic Phase of Sleep (RPS): This phase always follows a SPS and never appears immediately after wakefulness. It starts suddenly and is distinguished by a fast corticomesodiencephalic activity of low volt age, identical to that of wakefulness, while the rhinencephalic formations show a slow rhythmic activity, identical to that which has been described during arousal at this level (Green and Arduini 1954). Such a rhythmic activity has also been recorded at the cingulate gyrus, the septum, the posterior hypothalamus, the central grey matter of the mesencephalon and the interpeduncular nucleus. At the same time, a 6-8/sec spindle-like activity appears at the level of the pontile RF. The auditory cortical and reticular evoked responses undergo a marked reduction in amplitude compared with that in the waking state or during SPS. This phase lasts about 10-15 min and is accompanied immediately and constantly by total disappearance of EMG activity in the recorded muscles. It is periodically repeated during behavioral sleep, with intervals of 20-30 min ( Fig. 8). During that phase, the posture of the animal is the same as that found in profound sleep. Postural muscles are completely atonic, the nictitating membranes almost entirely cover the pupils which are miotic, while the eyeballs are frequently shaken by short rapid movements . Movements of the vibrissae and, more rarely brief jerks of the jaws and the tail can also be observed. Cardiorespiratory variations are consistently observed: breathing becomes irregular, more superficial and quicker than during SPS, while the heart rate is slowed down, or more rarely accelerated.
That this phase of sleep is more profound than SPS is supported by the following arguments:
- (i) An auditory stimulation, insufficient to produce arousal, causes the spindle and slow wave stage to reappear.
- (ii) The arousal threshold measured in decibels for an auditory stimulation, is higher during this phase than during SPS.
- (iii) The behavioral threshold of arousal obtained by direct electrical stimulation of the mesencephalic RF increases by 200 300 per cent compared to that of SPS (Jouvet et al. 1959b; Hara et al. 1960; Rossi et al. 1961).
This increase of the threshold of arousal, associated with the electrical activity of subcortical regions and with the total disappearance of the muscular tone and the somatic or vegetative phenomena permit the detection of the periodical appearance of RPS in animals with brain stem transection at several levels.
Part 1 : Experimental results obtained on cats
The rhombencephalic phase of sleep
Results
II. The neural structures responsible for RPS
A. The neural structures necessary and sufficient for the periodical triggering of the RPS
(i) The cerebellum, whose action might be strongly suspected in view of the total atony during RPS, is not involved since typical RPS appears in totally cerebellecto mized cats. In these animals, the alpha-type rigidity which distinguishes the post-operative period is abolished during RPS.
(ii) Total removal of the neocortex produces a surprising alteration in the electrical activity recorded at the level of the subcortical structures. In fact, there is a permanent lack of spindles and slow waves during the survival period of the animal (up to 6 months). Mesodiencephalic structures continuously exhibit a fast low voltage activity. However, the decorticate cats exhibit two different postures during sleep. There are short periods during which the eyes are closed. At this time, there are high voltage spikes in the limbic system. On the other hand the RPS, which constitutes 70-80 per cent of behavioral sleep, is shown by phenomena identical with those of the intact animal: appearance of "spindles" at the level of the pontile RF, theta rhinencephalic activity, disappearance of EMG activity, cardiorespiratory alterations, and appearance of eye movements almost similar to those of normal cats (Fig. 2).
(iii) Brain stem transection: chronic pontile or mesencephalic animal. The electrical activity of the cortical and diencephalic formations situated rostrally to the section, continually exhibits the classical appearance described at the level of the cerveau isole (Bremer 1935), i.e. a continuous mixing of spindles and slow waves, whatever the state of wakefulness (Fig. 3).
Behind the section, the mesencephalic activity constantly remains rapid during wakefulness. The periods of behavioral sleep are accompanied by the appearance of "spindles" at the level of the pontile RF and a complete disappearance of EMG activity in the neck. The latter change is especially remarkable if one compares it to the greatly increased muscular activity during wakefulness (decerebration hypertony). During these periods, the eye movements are slower and less frequent than in decorticate or normal animals, while the nictitating membrane is relaxed. Finally, important cardiorespiratory variations are observed. The duration of these phases is similar to that observed in intact animals, i.e. 10-15 min. The interval between phases is longer: 40-60 min. The threshold of behavioral arousal obtained by reticular electrical stimulation (shown by an increase in hypertony and midriasis) is also increased by 200 per cent during RPS compared to that observed during wakefulness. The same phenomena were observed in pontile cats in which all the brain rostral to the pons was removed (Fig. 4). In these animals the periodic appearance of RPS was similar to the "sudden postural collapse" observed by Bard and Macht (1958) in chronically decerebrate cats. These facts demonstrate that the neural structures responsible for the triggering of RPS are located behind the mesencephalon.
The determination of the posterior boundary of these structures has not been easy since it is very difficult to keep alive for several days an animal whose brain stem is transected behind the pons. Nevertheless, two cats survived for 1 week after a total removal of the cerebellum and a total transection of the brain stem made dorsally at the level of the caudal two-thirds of the nucleus reticularis pontis caudalis and ventrally at the limit between the pons and the trapezoid bodies (Fig. 5). In such a preparation two phenomena in particular appeared: behind the section there was no periodic variation in muscular activity nor in cardiac or respiratory activity (Fig. 6) analogous to that shown in the mesencephalic or pontile animals. The EMG activity remained constant throughout the survival period of the animal. Thus, medullary and spinal structures cannot trigger any periodical behavioral RPS when they are separated from the pons. Contrariwise, rostral to the section, very often, the cortical activity is fast, and is similar to the cortical activity described in the medio-pontine pretrigeminal preparation (Batini et al. 1959a, b, c).
The neural structures responsible for RPS are thus situated ahead of a pre-bulbar transection and behind a prepontine section. These structures must, therefore, necessarily be found at the level of the pons. For this reason limited lesions have been made at the level of the pontine reticular formation in order to suppress selectively the EEG and the behavioral correlates of the RPS.
(iiii) Lesions of the pontine reticular formation. Destruction of nucleus reticularis pontis caudalis suppressed the appearance of RPS in six cats (Fig. 7).
These cats were able to stand up and walk, and they could feed themselves. Some abnormality of behavior appeared in each of them, 3 or 4 days after the lesion. Periodically, they would have a fixed gaze with the head up and pupils dilated; and they would reach out with their paws as if attemping to touch an object. Such periodical "hallucinatory-like states" became more and more frequent throughout the survival period (up to I month).
In these cats, the cortical EEG was predominantly fast and the dorsal hippocampus showed a theta activity during the first days. On the 3rd day, periods of spindles and slow waves appeared during 50-60 per cent of the recorded time. The EEG and behavioral arousal were normal. On no occasion, in spite of continuous recording both day and night, was RPS observed: no fast EEG activity occurred during behavioral sleep and there was no disappearance of the EMG (Fig. 8). Nevertheless, in four cats, some RPS reappeared. But it was of very short duration (2-3 min) and had the same periodicity as before the coagulation of the pons. Its duration was only two per cent of the recorded time (compared with 25 per cent before the operation).
Thus, the destruction of the nucleus reticularis pontis caudalis suppresses the RPS. For this reason, we proposed the term RPS to describe the sleep stage characterized by fast EEG activity. This name seems more appropriate than "Paradoxical Phase" which we proposed earlier (Jouvet et al. 1959b).
B. Cerebral tracts responsible for the fast cortical EEG during RPS.
The location of the structures responsible for the triggering of RPS being established at the level of the nucleus reticularis pontis caudalis, circumscribed coagulations of the brain stem have been carried out, rostral to the pons, in order to suppress only the fast cortical and the theta hippocampal activity which are so characteristic of the RPS.
(i) Lesions of the lateral parts of the brain stem which destroy the specific ascending pathways and leave intact the mesencephalic reticular formation and the ventral part of the brain stem do not change the EEG correlates of SPS or of RPS, nor their behavioral correlates. Thus, such lesions do not affect the ascending pathways responsible for the fast cortical activity during RPS.
(ii) Lesions of the rostral part of the mesencephalic tegmentum (which leave intact the ventral part of the mesencephalon) suppress the low voltage fast cortical activity during arousal induced by nociceptive stimuli or by stimulation of the reticular formation behind the lesion (Moruzzi and Magoun 1949). Nevertheless, such lesions do not eliminate the possibility of fast cortical activity during RPS. This fact suggests that the cerebral tracts responsible for the cortical "activation" during sleep are necessarily different, at least in part, from the ascending activating reticular system which is responsible for cortical "arousal" (Moruzzi and Magoun 1949).
(iii) Lesions carried out at the level of the medial part of nucleus reticularis pontis oralis, interpeduncular nucleus and central grey matter, subthalamic region (medial hypothalamus, lateral liypothalamus, medial forebrain bundle) and septum, suppress, totally or in part, the low voltage fast cortical activity and the theta hippocampal activity during RPS without alteration of the behavioral aspects of this phase of sleep (Fig. 9). Such lesions, nevertheless, do not suppress the cortical arousal during wake fulness. Thus, lesions carried out at the level of the "limbic midbrain circuit" (Nauta and Kuypers 1957; Nauta 1958) suppress fast neocortical activity characteristic of RPS. It is possible that ascending corticopetal pathways coming from the nucleus reticularis pontis caudalis may take, at least in part, the limbic midbrain circuit (Fig. 10).
Part 1 : Experimental results obtained on cats
The rhombencephalic phase of sleep Results
III. Structures responsible for somato-vegetative phenomena
The appearance of typical RPS in the cerebellectomized animal rules out any possibility of the cerebellum playing a part. Therefore, the somato-vegetative phe nomena associated with this phase may be explained by the active intervention of the bulbar inhibitory RF, which is known to exert a general control on muscular tone and acts on the alpha-type as well as on the gamma-type rigidities (decerebellation and decerebration rigidities: Magoun and Rhines 1946; Magoun 1950). Respiratory and cardiovascular centers are also known to exist at the level of the pons and their intervention might explain the characteristic variation in respiratory and cardiac rhythms.
Part 1 : Experimental results obtained on cats
The rhombencephalic phase of sleep Results
IV. Mechanisms of the Rhombencephalic Phase of Sleep
This is an active phenomenon as it is possible to trigger off the appearance of RPS by stimulating the pontile RF of the intact animal, provided that stimulation occurs during SPS ( Fig. 11).
In mesencephalic cats, stimulation of this same zone is also capable of triggering periods of "Rhombencephalic Sleep". Phases of 15 min duration have thus been obtained after stimulations lasting 1-2 sec. After the animal has spontaneously awakened, a refractory period is observed, during which an identical stimulus determines a hypertonic phase accompanied by agitation. Therefore, it is never possible to trigger several periods of rhombencephalic sleep successively. An interval of 15-20 min must be allowed to elapse before a new stimulation can produce a new phase of sleep. In some animals, sleep has been obtained by summation of stimuli of long duration (10-20 sec) and in some cases a latent period of 30-60 sec was observed between the end of the stimulation and the beginning of sleep.
Atropine (0.2-0.3 mg/kg) considerably reduces the duration of RPS or even stops it from appearing, particularly in mesencephalic animals. On the other hand, the injection of cholinergic drugs (eserine) produces longer RPS although their frequency is not increased.
These facts are difficult to explain as a whole through purely neuronal mechanisms. They lead us to hypothesize the existence of a neurohumoral mechanism which would "discharge" periodically during behavioral sleep but which could not be brought into play until a sufficient "stock" of neurohormones has been accumulated.
Part I : Experimental results obtained on cats
The rhombencephalic phase of sleep Results
V. Ontogenesis of the RPS
The study of sleep in kittens (Jouvet et al. 1961b) shows that RPS is the first pattern of sleep observed after birth and confirms the duality of the neural structures responsible for the fast cortical activity during RPS and arousal. Indeed, during the first week after birth, behavioral RPS with total disappearance of the EMG of the neck, rapid eye movements, acceleration and irregularity of respiration and decrease of the heart rate appears during approximately 40 per cent of the record and represents almost the totality of behavioral sleep. At this time, there is no alteration of the cortical activity which remains the same whatever may be the state of wakefulness (very low voltage flat EEG with some regular 12-15/sec spindling activity; see Fig. 12).
Around the end of the second week, low voltage fast EEG activity appears during the RPS. However, at this stage the cortical activity remains slow (6-8/sec) during arousal and some short periods of slow EEG sleep (without EMG activity but also without rapid eye movements) begin to appear.
During the 3rd week only, tonic cortical arousal appears, almost at the same time that a typical slow phase of sleep (with EMG activity) begins to be recorded.
At the end of the 2nd month, the EEG activity of the kitten is similar to the adult cat with its three classical patterns: arousal, SPS with EMG activity and RPS.
Thus, during maturation and the progression of synaptic neuronal and glial organization of the cortex, the cortical EEG activity is modified at first by the ponto limbic system during the periodical RPS and secondly only by the reticular activating system whereas a phase of slow sleep without EMG activity, which is very exceptional in the adult cat, develops before giving way to the telencephalic phase of sleep ( Fig. 13).
The assembling of these results permits us to conclude that RPS depends on the periodical activity of a system whose structural organization may be summarized as follows: the integrity of a neuronal pool situated at the level of the nucleus reticularis pontis caudalis is necessary for the periodical appearance of RPS. This "center" is connected with the cortex through ascending pathways different from the reticular activating system. These pathways, going through the ventral mesencephalon, the subthalamic region, probably at the level of the midbrain limbic circuit, are specially connected with the limbic system. The descending pathways, coming from the nucleus reticularis pontis caudalis and responsible for the behavioral aspects of RPS, are probably related with the ponto-bulbar inhibitory reticular formation. The mechanisms of the physiological triggering of RPS are still unknown, but some results suggest that this phase depends upon a neurohumoral mechanism.
Part II : Clinical investigations in man
A. Normal subjects
We have carried out 20 records of sleep (from 10 p.m. to 6 a.m.) on six adult subjects (two men and four women, 20-35 years old) and three children (16 months, 22 months, 3 years). The EEG from the scalp was recorded continuously together with the movements of the eyes by means of electrodes placed in the vicinity of each eyeball. The EMG activity from the region of the back of the neck or from the external sternomastoid muscles, the EKG and the respiratory movements were also recorded with the EEG machine. The threshold of arousal was tested by auditory stimulation carried out by a loudspeaker triggered by a stimulator of variable voltage. During arousal produced in different stages of sleep, the subject was asked whether he remembered dreaming.
It is quickly apparent in these records that the EMG index is poor in man, at least with surface electrodes placed on the neck, since the EMG of both neck muscles and the sternomastoids disappear during the first stages of falling asleep, upon assuming the position of rest and sleep in man.
We shall adopt the terminology of Dement and Kleitman (1957a) on the subject of the different stages of sleep and we shall insist only on the phase of sleep characterized by rapid eye movements (REM).
Stage I is observed immediately upon falling asleep and may be very short in certain subjects. The threshold of arousal is very low. It is characterized by a low voltage relatively fast activity and an absolute absence of spindles or K-complexes. Slow pendular lateral movements of the eyes are observed at a frequency of about 10-20/min (Aserinsky and Kleitman 1955), and persist during the first three stages of sleep.
Stage II is characterized by the presence of spindle activity appearing on a back ground of fast activity and of low voltage. Sometimes theta activity may also appear. It is in this stage that K-complexes occur in the anterior and vertex regions.
Stage III is intermediate, characterized by the appearance of slow waves of high voltage associated with spindles.
Stage IV is characterized by the presence of diffuse delta waves of large amplitude, without spindles. During these last three stages the respiration is regular and slow and the heart rate decreases slowly.
The stage of sleep with REM. This stage has specific EEG and behavioral patterns which permit differentiation of it from the other stages of sleep (see Fig. 14 and 15). From the point of view of the EEG at the level of the occipItal. region, there are usually bursts or trains of alpha waves. These waves are often 1 or 2 c/sec slower than the waking frequency. The bursts of alpha waves are often "blocked" by REM. Besides these waves, the EEG is characterized by a low voltage activity without any rhythm. In some subjects, 20-25/sec waves are seen in the frontal region. There are no spindles or K-complexes spontaneously or induced by external stimuli. All these characteristics, however, may be observed during stage I of sleep.
But a very constant and specific activity is observed which permits a distinction between this stage of sleep and stage l ( Fig. 14). It consists of bursts of sharp waves, 2-3/sec in frequency. They have a triangular saw-tooth form and are rather narrowly localized to the central region. Their amplitude is maximal at the vertex, anterior parietal and anterior temporal leads. We have not observed them at the frontal or occipItal.regions.These waves occur in bursts of several seconds and usually immediately precede and sometimes overlap REM. They can last continuously, in some subjects, for some minutes. We have never observed such waves in other than the REM stage of sleep.
From the point of view of behavior, rapid eye movements during sleep have been described by Aserinsky and Kleitman (1955) and by Dement and Kleitman (1957a,b). They are very characteristic and constant and we have observed them in all subjects every night. We have found them in a 16-month-old baby and in a 3-year-old child who was blind since the age of one. These movements, bilateral and synchronous, with the eyelids half closed, are quite easily seen since the muscular tonus of the orbicularis decreases and the lids open enough to allow the white sclera to show. They resemble the scanning movements that might be executed by an awake subject while watching some activity taking place in his field of vision. These REM appear in clusters or bursts, but sometimes they may be almost continuous for some minutes. At the same time it is common to see discrete movements of the corners of the mouth and of the fingers. Respiratory variations are constant in all subjects: irregularity, decrease of amplitude and acceleration. It is not infrequent to observe a complete apneic pause at the beginning or at the end of the REM period of sleep.
The variability of the respiratory rate seems to be, together with REM, the best behavioral sign of this stage of sleep. The variations in rhythm of the heart are less constant. We have observed more often a slowing of the pulse. But in the same subject, during the same night, slowing, acceleration or no change may be observed during the REM period. This stage of sleep occurs periodically during the night ( Fig. 15), with a duration of 20-30 min. Usually the last period is the longest one. In adult subjects, the total duration is 20-25 per cent of the night's sleep. In children under 3, they represent 25-35 per cent of the duration of behavioral sleep. During this stage, the threshold of arousal by auditory stimuli is greatly augmented. We have found it 3 to 4 times higher than during stage II, 1.5-2 times higher than during stage III and IV ( Fig. 15).
REM periods and dream recall. We will discuss here only those dreams which could definitely be recalled by the subjects. Among 45 arousals during or immediately after a REM period, there were 31 reports of dreams (65%). On the contrary, among 40 arousals during stage I, II, III, or IV there were only three dream recalls (8%). This result confirms the findings of Dement and Kleitman (1957a, b) and strongly suggests that the REM stage of sleep signals the presence of dreaming in the human.
Part II : Clinical investigations in man
B. Patients with brain lesions
The structural analysis of the human EEG suffers from a major handicap: the impossibility of producing experimental lesions. However, in certain cases, diseases or trauma may bring about systematic nervous changes comparable to those of an experiment. For this reason, we have studied patients in a state of prolonged coma (from 6 months to 5 years). Polygraphic recordings (EEG, EMG, eye movements, EKG and respiration) were carried out both day and night in order to analyse the different patterns of sleep. The results of this study (Pellin 1960; Jouvet et al. 1960, 1961a), which are almost similar to the results of animal experiments, may be summarized as follows:
(i) Syndrome of chronic decortication. Five adult patients had a prolonged altered state of consciousness due to white matter degeneration. After a severe head trauma, these patients were bed-ridden for several months to several years. They had no visual or auditory perception and could not obey simple commands. They opened their eyes and stared aimlessly for long periods during the day. Strong nociceptive stimuli would nevertheless induce a true painful countenance with midriasis and polypnea. All patients were very spastic and exhibited a characteristic rigidity.
The findings at autopsy were remarkably similar in all cases and have been described elsewhere in detail (Pellin 1960; Trillet 1961). There were no focal lesions in the brain stem. Microscopically, there was a severe diffuse degeneration and often a necrosis of the cerebral white matter, associated with a degeneration of descending tracts ( Fig. 16). Such a traumatic encephalopathy is very similar to the cases reported by Strich (1957). Thus, in all these cases, the corticopetal and corticofugal fibers were almost all destroyed at the level of the cerebral white matter and the final physiological result may be considered as a decortication.
In these patients, the polygraphic patterns of the sleep-wakefulness rhythm were similar ( Fig. 17 and 18). During wakefulness, with eyes open, there was a large amount of EMG activity at the level of the flexor muscles of the upper extremities (biceps) due to the hypertony of decortication. There were also many muscle artifacts in the EEG due to incessant chewing movements. The respiration had a periodic pattern. The EEG was characterized by a low voltage theta rhythm which was not altered by sensory stimuli.
When the patients were left undisturbed in a silent and dark room, this pattern of wakefulness was periodically interrupted (2-3 times per h) by short periods of behavioral sleep whose polygraphic patterns were very similar to the RPS observed in decorticate cats. During this behavioral sleep, the EMG activity totally disappeared, while synchronous rapid bilateral eye movements occurred. Respiratory variations were quite clear cut. The periodic breathing observed during wakefulness gave way to deep and steady breathing. There were also cardiac rhythm alterations ( Fig. 18).
During sleep, there were never slow waves or spindles in the EEG. The electrical activity remained with the same frequency as during wakefulness but with some decrease of amplitude.
During these periods of sleep, which lasted for 7-10 min (their total duration was about 25 per cent of the recorded time), the thresholds of arousal were strongly enhanced. It was almost impossible to arouse the patients by auditory stimuli capable of inducing some primitive orientating reaction during wakefulness. Only nociceptive stimuli were effective.
(ii) Syndrome of chronic decerebration. Two patients had a decerebrate state during 3 months after head trauma with decerebrate rigidity, "unconsciousness", and an almost permanent waking state.
In such cases, there were short periods of behavioral sleep with slow waves. But an intense EMG activity ( Fig. 19) persisted through these periods in the extensor muscles of the upper and the lower extremities (triceps brachialis and quadriceps cruralis). Only extremely short periods (2 min) of disappearance of EMG activity associated with REM were observed. Their total duration did not exceed 2 per cent of the recording time.
The autopsy showed localized infarction at the level of the reticular formation of the pons, the brachium conjunctivum and the inferior part of the mesencephalon.
Discussion
A. Differences in electrical activity
The cortical activity in the cat during RPS is very similar to the arousal reaction. In man, however, the EEG during REM is different from the EEG recorded during wakefulness (arousal reaction with opening of the eyes). Nevertheless, the alpha or subalpha rhythm observed in the occipItal. region during sleep is similar to the rhythm recorded in awake humans with the eyes closed.
B. Similarities of the behavioral aspects of sleep
The REM recorded in man are very similar to those observed in the cat. They are accompanied by small movements of the extremities in both species and by movements of the angles of the lips in man and of the whiskers in the cat. The vegetative mani festations are also similar.
The similarity between the disappearance of muscle tone both in cat and man is very remarkable in the cases of pathological hypertony (decerebrate rigidity in the cat, decorticate rigidity in man). In normal humans, the total atony occurring during dreaming is not easy to determine since it is preceded by the hypotony of the first stages of sleep.
However, the total atony during nightmares was recognized by Kouretas and Scouras (1932) and recently R. Berger (1961) has observed a striking decrease of the muscle tone of extrinsic laryngeal muscles in normal humans at the onset of each phase of sleep with REM whereas EMG activity persisted during the other stages of sleep. This finding thus confirms that, in normal humans, sleep with REM is ac companied by a total decrease of muscle tone as in the cat.
That the REM periods of sleep are more profound than other stages in normal humans is supported by the rise of the arousal threshold to auditory stimulation. Whatever may be the neural mechanisms responsible for this increase of the depth of sleep, both RPS in the cat and sleep with REM in man stand apart as qualitatively different phenomena which appear periodically during sleep.
C. Structural similarities
Certainly, pathological brain lesions observed in man are not yet as numerous as the experimental lesions carried out in cats and only a very superficial analogy may be obtained.
Nevertheless, the patterns of sleep observed in both decorticate cats and humans are very similar, and the almost total disappearance of the phase of sleep with REM and decrease of muscle tone in humans with pontile lesions is analogous with the results obtained after coagulation of the pons in the cat.
D. Other functional aspects
In cats, the coagulation of the nucleus reticularis pontis caudalis suppresses RPS and produces the periodical appearance of some "hallucinatory behavior" after 3 or 4 days.
In man, sleep deprivation also produces periodic visual hallucinations and tactual misperceptions after the same period of time (Katz and Laudis 1935; Bliss et al. 1959; Brauchi and West 1959). The experiment of "dream deprivation" during two nights made by Dement (1960) may not have been long enough to induce such hallucinations.
It is certainly impossible to compare the subjective experiences of cats and humans. Nevertheless, it must be remembered that many psychoses are preceded by some insomnia. It is possible that dysfunction of the phase of sleep with REM could be responsible for the appearance of some psychotic states.
Thus, this short review of the differences and of the similarities between the RPS in the cat and the sleep period with REM in man permit the conclusion that it is very likely that dreaming occurs during a stage of sleep analogous to the RPS (it is, of course, impossible to state that the cat dreams during the RPS).
Dream activity would thus appear to be the subjective result of the cerebral mechanisms occurring during RPS. It is a periodic phenomenon elosely related with the decrease of muscular tone and the alteration of vegetative functions (which are not however related to it in a reciprocal fashion since they appear in decorticate subjects). Dreaming would thus depend upon the periodic coming into play, during sleep, of a neuronal pool situated in the reticular formation of the pons, related rostrally with the limbic system by ascending pathways different from those of the reticular activating system.
A more detailed analysis of the mechanisms and of the functions of dreaming is difficult and more experiments are needed in order to shed more light upon the mysterious activity occurring in the brain during dreams. Many problems are still not solved:
Why is the RPS, contemporaneous with an increase of the unit activity at the cortical and reticular levels (Evarts 1961; Huttenlocher 1961), always associated with a total decrease of the muscular activity? What is the function of this cerebral dream activity associated with muscular sleep which periodically interrupts the slow wave stages of sleep? On the other hand, this last stage does not seem to be associated with any subjective activity but is associated with some muscular activity.
The mechanisms of REM observed in the human during RPS, and which seem to be closely related to the visual scenery of dreams (Dement 1955) are also difficult to explain. Indeed, such eye movements are observed in a baby, in a blind child, in adult patients apparently unconscious for many years and in decorticate cats. The memory processes which are related with these scanning movements must be necessarily located in limbic or brain stem areas.
Dreaming, which periodically occurs during a quarter of our night's sleep, has for centuries aroused curiosity, religious or metaphysical speculations, and sometimes fear. For the neurophysiologist the key to understanding dreams has not yet been found and the functions of dreaming are still mysterious. However, as irrational as the contents of our dreams may be, their visual contents are integrated into the unity of our organism, since they are dependent, as are other vegetative and homeostatic mechanisms, on neurons located in the pons.
Summary
Recollection of dreams occurs when a sleeping subject is aroused during the stage of sleep with low voltage EEG activity associated with rapid eye movements. This fact has led to the hypothesis that such a stage of sleep is associated with dreaming.
A similar stage of sleep with fast cortical activity and rapid eye movements has been described in the cat. The neurophysiological mechanisms underlying this stage have been studied in chronic intact, decorticate, mesencephalic and pontile cats. The results show that the periodical fast cortical activity during sleep (Rhombencephalic Phase of Sleep: RPS) is dependent upon triggering of a system located at the level of the nucleus reticularis pontis caudalis in the pontine reticular formation. This system controls the somato-vegetative phenomena which are highly characteristic of the RPS (disappearance of all muscular tonic activity even in the cases of decerebration and decerebellation hypertony, variation in respiratory and cardiac rhythms). The fast cortical and the slow 5/sec rhythmic hippocampal activities occurring during RPS are not suppressed by the interruption of the mesencephalic reticular formation, but are suppressed by lesions of the ventral mesencephalon, the hypothalamus and the septum.
RPS is more profound than the phase of sleep associated with slow cortical activity since the threshold of arousal in the former is higher than in the latter. RPS can be triggered off in animals by stimulating the brain stem and some results suggest that a neuro-humoral mechanism may be responsible for its periodical appearance. In kittens, RPS is the first stage of sleep to appear after birth.
Investigations on normal human subjects confirm that dreaming is associated with the stage of sleep accompanied by rapid eye movements. 3/sec "saw-tooth" waves, occurring at the vertex are specific to this stage. Results obtained on chronic decorticate and decerebrate human subjects have confirmed the main results obtained in the cat, and lead to the conclusion that the stage of sleep with rapid eye movements in humans is similar to the RPS and depends upon the same neural structures.
Such results suggest that dreaming occurs periodically during sleep when a ponto limbic system is brought into play, probably by a neuro-humoral mechanism.
Fig. 1 : The two phases of sleep in an intact cat
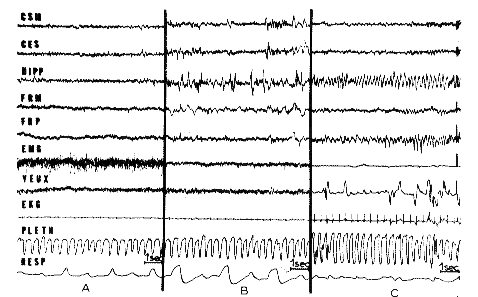
- A. Arousal: Fast cortical, hippocampal and reticular activity
- B. "Slow Sleep": Slow waves and spindles at the cortical and reticular level, persistence of EMG activity of the neck muscles (EMG).
- C. Rhombencephalic Phase of Sleep (RPS): Fast cortical and mesencephalic reticular activity similar to the arousal. Theta rhythm at the hippocampus (Hipp). Theta rhythm and spindles at the pontine reticular level. Total disappearance of the EMG activity. Rapid eye movements (Yeux). Augmentation of the plethysmographic index of the anterior leg. Irregularity of the respiration.
- CSM: Sensorimotor cortex;
- CES: Ecto-sylvian cortex;
- HIPP: Ventral hippocampus;
- FRM: Mesen cephalic reticular formation;
- FRP: Pontine reticular formation;
- EMG: Electromyogram from neck muscles;
- Yeux: Ocular movements;
- EKG: Electrocardiogram;
- Pleth: Plethysmogram of a leg;
- Resp: Respiratory movements.
From Jouvet (1962), with kind permission of Arch. Ital. Biol. (full-text)
Fig. 2 : RPS in a neodecorticate cat
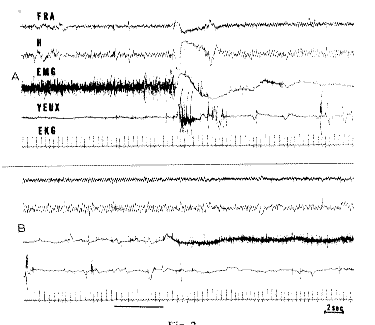
A. A theta rhythm appears at the level of the ventral hippocampus (H) and of the medial ventral reticular formation (FRA). At the same time, the EMG of the neck muscles disappears and rapid eye movements occur with some blinking. B. 40 sec after A: A very loud noise (horizontal line) arouses the cat. Scale: 2 sec.
Fig. 3 : Arousal and RPS in a chronic mesencephalic cat
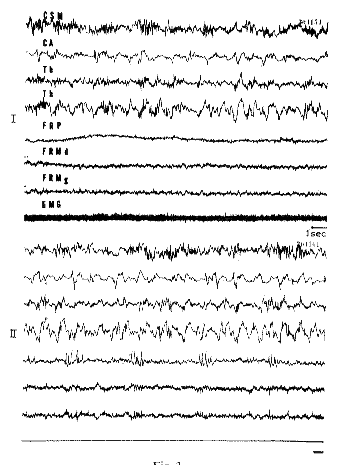
Total transection of the brain stem at the frontal plane A8.
I. Behavioral arousal. Spindles and slow waves at the cortical and thalamic level (Th), in front of the section. Low voltage fast activity in the pontine and mesencephalic reticular formation, behind the section. EMG activity of the neck muscles.
II. RPS. There is no change of the EEG activity rostral to the section (since the ponto-limbic pathways are interrupted). Behind the section "spindle" activity in the pontine reticular formation (FRP). Total disappearance of the EMG of the neck. FRM d and g: Bipolar recordings from the right and left part of the mesencephalic tegmentum.
Scale: 1 sec.
From Jouvet (1962), with kind permission of Arch. Ital. Biol. (full-text)
Fig. 4 : Sagittal section of the brain stem of a pontile cat
There is an electrode trace in the cerebellum and the caudal part of nucleus reticularis pontis caudalis.
Fig. 5 : Sagittal section of the brain stem in a retro-pontile cat

Note the oblique transection of the brain stem caudal to the pons. Electrode trace in the nucleus reticularis gigantocellularis. Loyez stain.
Fig. 6 : Somatic and vegetative patterns in pontile and retro-pontile cats
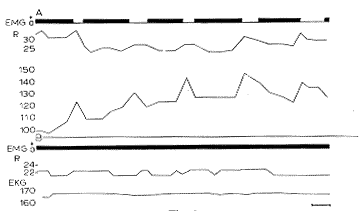
A. Pontile cat (see Fig. 4). Periodical appearance of 5 RPS (27% of the time) during which there is a total decrease of the EMG of the neck (in black), acceleration of respiration (upper line) and of the EKG (lower line).
B. Retro pontile cat (see Fig. 5). Permanent "vigilance" of the somato-vegetative activity. No disappearance of the EMG, nor variation of respiration or EKG. Each recording was made 5 days after the lesion.
Time scale: 10 min.
Ordinates: respiration and heart rate (per min).
Fig. 7 : Coagulation of the nucleus reticularis pontis caudalis suppresses the RPS
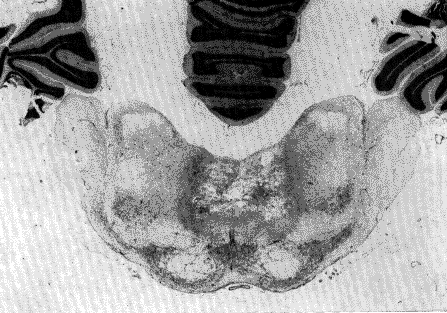
This animal was sacrificed 60 days after the lesion. It did not have RPS during 45 days. It had a normal EEG and behavioral arousal or SPS. It showed also periodical apparent hallucinatory states during wakefulness. After 45 days, some brief RPS reappeared. Nissl stain.
Fig. 8 : Diagrammatic representation of the sleep-wakefulness rhythm
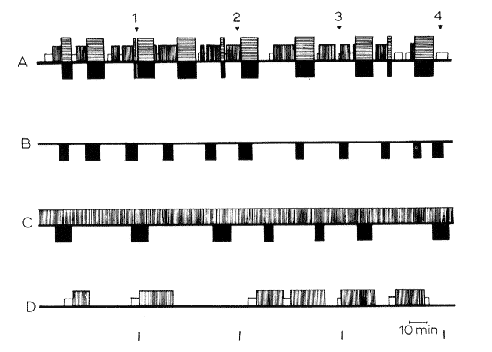
in (A) intact, (B) decorticate, (C) mesencephalic cats, and (D) cats with coagulation of the nucleus reticularis pontis caudalis. 4 h of continuous recordings were taken in each cat. In black: RPS with fast cortical EEG pattern in normal cats (horizontal hatching) and spindling activity at the pontine level, total disappearance of EMG activity, eye movements, in normal, decorticate and mesencephalic cats. In white: spindling activity on the cortex. Verfical hatching: slow waves and spindles at the cortical and diencephalic level. Horizontal line: arousal. Note the absence of SPS in decorticate cats, and the absence of RPS in the cat with pontine lesion. The slow EEG activity in C represents the "lethargic state of the brain "in cerveau isole' but not a true "slow sleep".
Time scale: 10 min.
From Jouvet (1961), with kind permission of J. and A. Churchill Ltd., London.
Fig. 9 : Absence of fast cortical activity
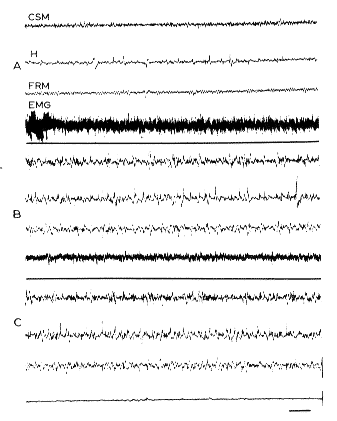
during RPS after total destruction of the septum. A. Arousal: fast cortical (CSM), hippocampal (H) and reticular mesencephalic (FRM) activity. B. SPS: spindles and slow waves, persistance of the EMG activity of the neck. C. RPS: there is a discrete activation at the cortical level, but some spiddles persist. No theta rhythm at the hippocampal level. Disappearance of the EMG
Scale: 2 sec, 50 microV.
Fig. 10 : Schematic representation of the neural structures responsible for RPS
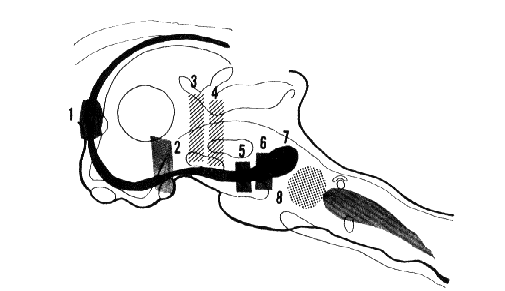
In dots (8). Nucleus reticularis pontis caudalis whose destruction suppresses RPS. In black. An ascending part of the limbic midbrain circuit with the "limbic midbrain area" of Nauta and Kuypers (1957). 1-2-5-6. Lesions of the septum, subthalamic region, interpeduncular region, and medial part of the anterior pontine tegmentum. These lesions suppress, totally or in part, the fast cortical activity and the theta hippocampal rhythm during RPS. 3-4. Lesions interrupting the ascending reticular activating system at the mesencephalic level. These lesions, which suppress cortical arousal, do not eliminate the possibility of a fast cortical activity during RPS. In grey. Ponto-bulbar inhibitory reticular formation which is probably responsible for the total atony during RPS.
After Jouvet (1962), with kind permission of Arch. Ital. Biol. (full-text)
Fig. 11 : Triggering off RPS in an intact chronic cat
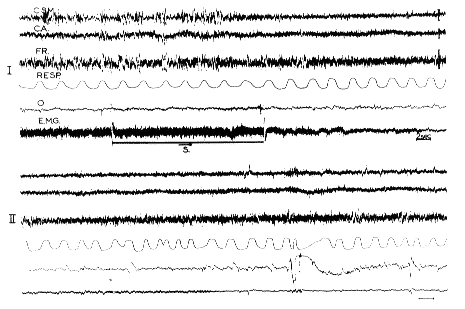
The horizontal line (S) signals the stimulation of the pontine reticular formation (300/sec; 1 msec pulses; 0.8 V; bipolar) for 11 sec during SPS. Note the appearance of fast activity at the cortical and reticular level, the total disappearance of the EMG activity of the neck muscles (EMG), the acceleration and irregularity of the respiration (Resp), and the appearance of rapid eye movements (O). This RPS lasted for 10 min. The same phenomenon was reproduced five times in this session. The two recordings I and II are continuous.
- CSM: Sensory motor cortex;
- CA: Acoustic cortex;
- FR: mesencephalic reticular formation.
Scale: 2 sec, 50 microV.
From Jouvet et al. (1960).
Fig. 12 : RPS in kittens
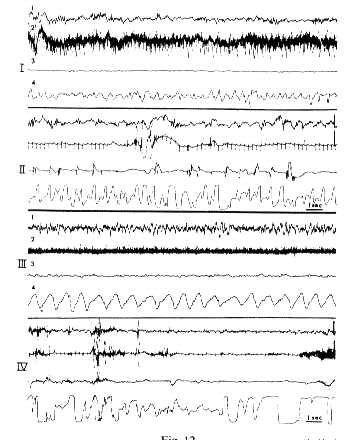
- I. 30 h after birth: Behavioral arousal.
- II. 2 min after I. RPS Disappearance of neck muscle activity. Eye movements, acceleration of respiration. The EEG is similar to I.
- III. Same kitten (21 days after birth). Behavioral arousal; slow 7/sec cortical activity.
- IV. 3 min after III. RPS;fast low voltage cortical activity. Disappearance of the EMG with some jerks.
- 1: Cortical EEG.
- 2: EMG of the neck.
- 3: Eye movements.
- 4: Respiration.
Scale: 1 sec, 50 ,microV.
From Jouvet et al. (1961b).
Fig. 13 : Diagrammatic representation of the evolution of the sleep-wakefulness rhythm during the first 2 months in kittens
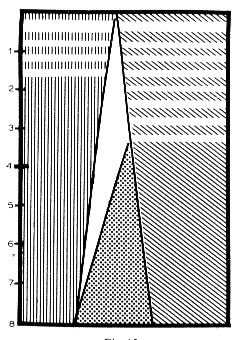
Ordinate: Time (in weeks).
Abscissa: Relative percentage of sleep vs. wakefulness, calculated from 6 h of continuous recordings every day.
Vertical hatching: Behavioral RPS.
White horizontal lines signal the period during which there is no fast cortical activity during RPS.
Oblique hatching: Behavioral arousal.
White horizontal lines signal the period during which there is no tonic cortical arousal.
In white: Sleep with slow cortical activity but without EMG activity of the neck and without REM.
In dots: Classical SPS with slow cortical activity and EMG activity.
Fig. 14 : EEG patterns of RPS in man
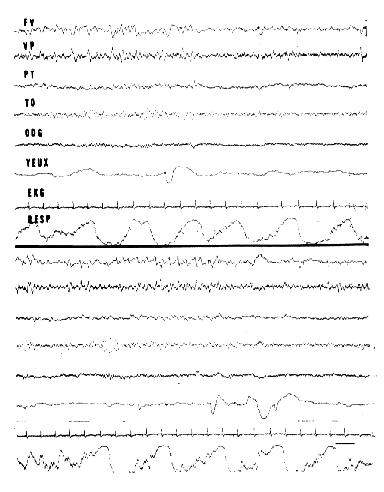
3/sec "saw-tooth" waves occurring at the vertex-frontal (FV), and vertex-parietal (VP) region, before REM (yeux). Alpha rhythm at the occipItal. region (TO-ODG). Irregularity of respiration. These two recordings have been made during two successive periods of RPS in the same night in a normal adu1t subject. This subject was aroused at the end of these RPS and remembered having dreamt.
Scale: 1 sec.
Fig. 15: Periodicity of RPS and "depth" of sleep
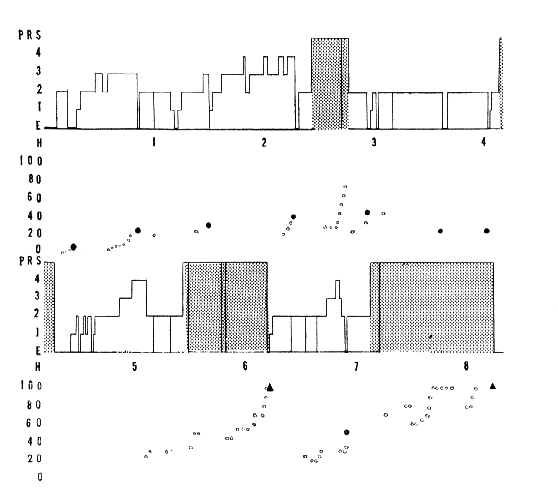
All night's sleep of 8 h 20 min duration in a normal adult. It is interrupted by four periods of RPS whose duration is 28 per cent of sleep.
Ordinate: E, arousal;
1-2-3-4, the four stages of sleep, and RPS (PRS).
Scale (in V) of the stimulator which triggered a loud speaker (1000 c/sec tone).
The white dots signal auditory stimulation which did not arouse the subject.
The black dots, auditory stimuli which did arouse the subject without recall of dream.
Black triangles, recall of dreams.
The two diagrams are continuous.
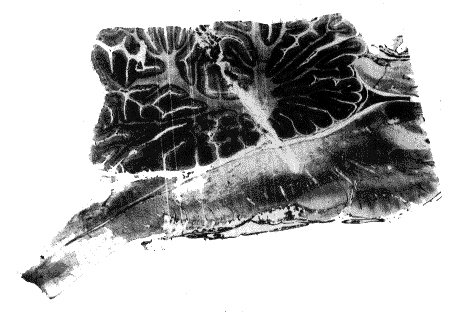
Fig. 16 : Brain lesion in a case of prolonged unconsciousness (syndrome of decortication)
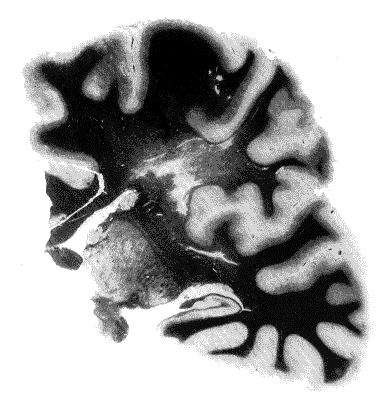
Coronal section through the left cerebral hemisphere (coloration of Woelcke). Severe and diffuse degeneration of the white matter with necrosis. There was a similar degeneration in the right hemisphere. The brain stem was intact.
Fig. 17 : Arousal and RPS in a syndrome of decortication
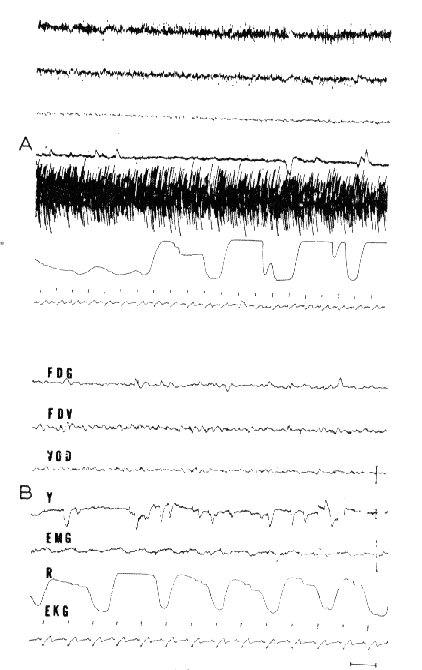
A. Arousal: Very low voltage EEG activity and considerable EMG activity at the frontal region and the biceps (hypertony of decortication). Periodic breathing.
B. RPS: Total disappearance of the EMG activity. Discrete rhythmic 3/sec slow waves at the vertex. Rapid eye movements (Y). Slowing of the EKG. Acceleration of respiration.
- FDG: right and left frontal;
- FDV: vertex-right frontal;
- VOD: vertex-right occipItal.
Scale: 1 sec, 50 microV.
Fig. 18 : Sleep-wakefulness rhythm in a syndrome of decortication
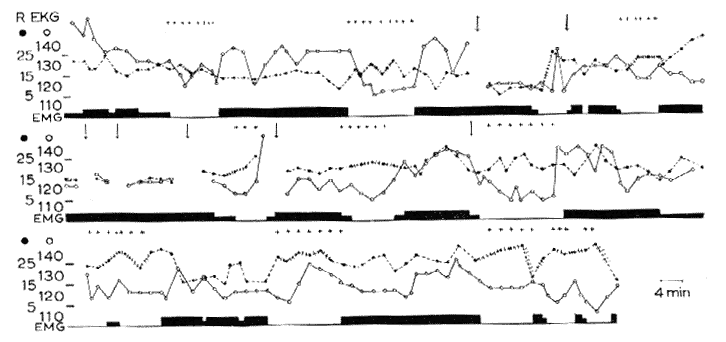
Continuous recordings during 8 h (from 9 p.m. to 5 a.m.). Vertical arrows signal brief interruption of the recording.
Abscissa: In black: EMG activity (biceps).
Black dots and interrupted line: respiration (per min). The irregularity of respiration is marked by small vertical lines on the respiratory curve.
White dots and solid line: Heart rate (per min).
Crosses: rapid eye movements.
Ordinate: Respiratory and heart rate (per min). Note the periodical appearance of RPS (25 per cent of the time) during which there are REM, cardiorespiratory variations and a total disappearance of the EMG.
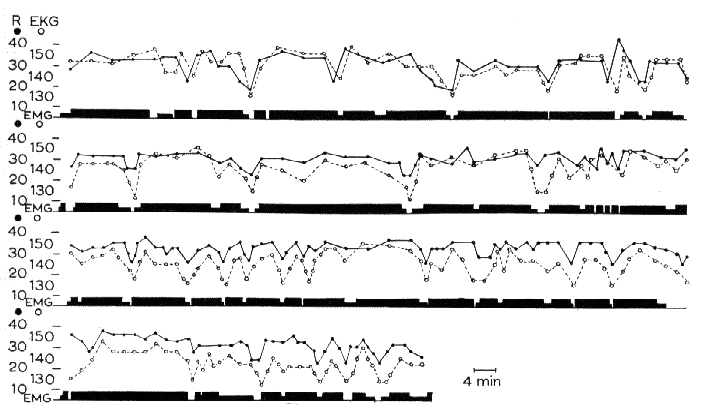
Fig. 19 : Permanent pattern of muscular activity in a syndrome of decerebration with pontine reticular lesion
Continuous recording during 9 h (from 10 p.m. to 7 a.m.). The EMG activity (triceps brachialis) persists almost throughout the night, with only very short periodical disappearance, accompanied with slowing of EKG (note the tachycardia). Three other similar recordings have been made on this subject. Time scale: 4 min Same legend as in Fig. 18.