Hyperoxia increases paradoxical sleep rhythm in the pontine cat
Isabelle Arnulf (a), Jean-Pierre Sastre (b), Colette Buda (b), Michel Jouvet (b)
Brain Research 807 160-166 1998
a UPRESS EA2397, Pitié-Salpétrière Hospital, Paris, France, b INSERM U480, CNRS URA 1195, Claude Bernard University, Lyon, France
Abstract
Pontine cat is an ectothermic preparation, whose central temperature can artificially be lowered from 36°C to 26°C; this gradual hypothermia is accompanied by a dramatic increase in paradoxical sleep (PS). Two main hypotheses might explain this result: executive systems of PS might be switched on gradually by cold-sensitive thermodetectors, whereas inhibitory monoaminergic mechanisms appear to be warm-sensitive. On the other hand, energy saving mechanisms peculiar to hypothermia might promote PS appearance. Indeed, in normal animals, PS is selectively suppressed both by hyperthermia and hypoxia. The inhibitory effect of hypoxia might explain why hypothermia, which protects the brain against hypoxic alterations, might facilitate PS. If this last hypothesis is correct, the putative increase in cerebral oxygen supply might increase PS. For this reason, we submitted eight pontine carotid-deafferented cats, kept at the same central temperature (34 ± 0.5°C: temperature clamp) to periodic hyperoxia (PaO2 = 58 ± 7 kPa) or room air (Pa02 = 17 ± 2 kPa) alternatively during 4- or 12-h periods. Hyperoxia induced an 85% increase in PS, mainly due to an increase in PS rhythm (PS cycle duration was 65 ± 4 min in normoxia and 45 ± 4 min in hyperoxia, p < 0.0001). In five animals, after hyperoxia, PS cycle returned gradually back to control values in 4 to 12 h. These findings show that PS is exquisitely sensitive to conditions that impair oxidative metabolism. The role of cholinergic executive PS systems as putative metabolic- sensitive neurons remains to be established
Keywords: Paradoxical sleep; REM sleep; Rhythm; Oxygen; Hypothermia; Hyperoxia; Acetylcholine
1. Introduction
In the chronic pontine cat, an ectothermic chronic preparation, paradoxical sleep (PS) is strongly dependent upon central temperature (CT). On the one hand, PS does not appear beyond 36°C, while the periodic decrease in CT below 36°C may induce sleep. On the other hand, progressive hypothermia, from 35°C to 25°C is accompanied by a dramatic increase in PS, from 2% at 36°C to 90% at 25°C [17-20]. This phenomenon was tentatively explained by differential heat and cold sensitivity of both inhibitory and executive mechanisms of PS or by energetic mechanisms. Monoaminergic systems located in the raphe and in the locus coeruleus, whose increased activity suppresses PS (for a review, see Ref. [16]) are activated by high CT [27]. On the one hand, cold-sensitive nerve cells, firing below 34°C, have been described in the pontine tegmentum [2] and it may be speculated that executive neurons of PS could be excited by low CT. On the other hand, in normal animals from various species, PS is selectively suppressed both by hyperthermia [21,22,24,36] and hypoxia [3,13,23,28]. This inhibitory effect of hypoxia might explain why hypothermia, which decreases cerebral metabolic rate [7] and protects the brain against hypoxic alterations [32,35] might facilitate PS. In fact, the oxygen (02) supply to the brainstem in a pontine preparation might be impaired, so that a relative tissular dysoxia could exist despite a normal arterial oxygen pressure (Pa02). If this hypothesis is correct, the putative increase in cerebral 02 supply might increase PS. For this reason, we submitted pontine cats, kept at the same CT just below PS threshold (34°C) (temperature clamp) to periodic hyperoxia. Our results demonstrate that hyperoxia increases PS by 85% mainly by increasing PS ultradian rhythm.
a UPRESS EA2397, Pitié-Salpétrière Hospital, Paris, France, b INSERM U480, CNRS URA 1195, Claude Bernard University, Lyon, France
Abstract
Pontine cat is an ectothermic preparation, whose central temperature can artificially be lowered from 36°C to 26°C; this gradual hypothermia is accompanied by a dramatic increase in paradoxical sleep (PS). Two main hypotheses might explain this result: executive systems of PS might be switched on gradually by cold-sensitive thermodetectors, whereas inhibitory monoaminergic mechanisms appear to be warm-sensitive. On the other hand, energy saving mechanisms peculiar to hypothermia might promote PS appearance. Indeed, in normal animals, PS is selectively suppressed both by hyperthermia and hypoxia. The inhibitory effect of hypoxia might explain why hypothermia, which protects the brain against hypoxic alterations, might facilitate PS. If this last hypothesis is correct, the putative increase in cerebral oxygen supply might increase PS. For this reason, we submitted eight pontine carotid-deafferented cats, kept at the same central temperature (34 ± 0.5°C: temperature clamp) to periodic hyperoxia (PaO2 = 58 ± 7 kPa) or room air (Pa02 = 17 ± 2 kPa) alternatively during 4- or 12-h periods. Hyperoxia induced an 85% increase in PS, mainly due to an increase in PS rhythm (PS cycle duration was 65 ± 4 min in normoxia and 45 ± 4 min in hyperoxia, p < 0.0001). In five animals, after hyperoxia, PS cycle returned gradually back to control values in 4 to 12 h. These findings show that PS is exquisitely sensitive to conditions that impair oxidative metabolism. The role of cholinergic executive PS systems as putative metabolic- sensitive neurons remains to be established
Keywords: Paradoxical sleep; REM sleep; Rhythm; Oxygen; Hypothermia; Hyperoxia; Acetylcholine
1. Introduction
In the chronic pontine cat, an ectothermic chronic preparation, paradoxical sleep (PS) is strongly dependent upon central temperature (CT). On the one hand, PS does not appear beyond 36°C, while the periodic decrease in CT below 36°C may induce sleep. On the other hand, progressive hypothermia, from 35°C to 25°C is accompanied by a dramatic increase in PS, from 2% at 36°C to 90% at 25°C [17-20]. This phenomenon was tentatively explained by differential heat and cold sensitivity of both inhibitory and executive mechanisms of PS or by energetic mechanisms. Monoaminergic systems located in the raphe and in the locus coeruleus, whose increased activity suppresses PS (for a review, see Ref. [16]) are activated by high CT [27]. On the one hand, cold-sensitive nerve cells, firing below 34°C, have been described in the pontine tegmentum [2] and it may be speculated that executive neurons of PS could be excited by low CT. On the other hand, in normal animals from various species, PS is selectively suppressed both by hyperthermia [21,22,24,36] and hypoxia [3,13,23,28]. This inhibitory effect of hypoxia might explain why hypothermia, which decreases cerebral metabolic rate [7] and protects the brain against hypoxic alterations [32,35] might facilitate PS. In fact, the oxygen (02) supply to the brainstem in a pontine preparation might be impaired, so that a relative tissular dysoxia could exist despite a normal arterial oxygen pressure (Pa02). If this hypothesis is correct, the putative increase in cerebral 02 supply might increase PS. For this reason, we submitted pontine cats, kept at the same CT just below PS threshold (34°C) (temperature clamp) to periodic hyperoxia. Our results demonstrate that hyperoxia increases PS by 85% mainly by increasing PS ultradian rhythm.
2. Materials and methods
2. 1. Surgical procedure
The effects of hyperoxia were studied in eight pontine cats, six males (C122, F122, J122, N122, P122, C123), two females (Z123, H124) weighing from 2-2.8 kg. All animals were treated according to the guidelines approved by the French Ethics Committee (Decree 87-848). Surgery was performed under deep pentobarbital anesthesia (Nembutal, 25 mg/kg i.v.) on the day immediately preceding the recordings (DO). Both carotids were ligated upstream from the carotid chemoreceptors. The calvarium was opened, the dura mater sectioned and the sagittal sinus ligated at both extremities. The brainstem was then transected with a knife in the stereotaxic coronal plane A2.5, just rostral to the pons. All cerebral tissues rostral to the transection, including cortex, thalamus, hypothalamus and pituitary gland, were removed by aspiration. The olfactory bulb was left intact (Fig. 1). Local bleeding was controlled by clipping the arteries and by applying local haemostatic gelatin. Bipolar electrodes were implanted in the olfactory bulb to record Adrian waves [1], in the orbits to record eye movements and in the abducens nuclei to record pontogeniculo-occipital waves (PGO). Electrodes were placed on the neck muscles to record the electromyogram (EMG) and on the chest to record the electrocardiogram (EKG). The calvarium was then closed and fixed with acrylic cement.
2.2. Temperature clamp
After surgery, the cats were placed in an incubator. A the istor probe was fixed in the colon to measure central temperature (CT). An automatic device maintained CT precisely around a fixed level (34.0 ± 0.5°C) by controlling the heating of the incubator (Ta = 26.6 ± 1.7°C). Electrophysiological activity was continuously monitored over four days on an 8-channel polygraph (ALVAR, REEGA, Paris, France), run at 30 cm/min during the day and at 60 cm/min during the night. Rectal temperature measured with thermistors was continuously recorded on a Linseis recorder (LINSEIS, Germany) together with the integrated activities of the EMG, PGO waves, and olfactory bulb.
When CT reached 34.5°C (fixed as maximal CT), the automatic device stopped the incubator heating until CT reached 33.5°C (fixed as minimal CT). At 33.5°C, the incubator was automatically switched on. The rate of cooling of the animal, i.e., the duration between maximal and minimal CT was measured on the Linseis recorder. This rate of cooling was used as an index of the residual thermogenesis.
2.3. Animal care
The cats were not given any substitutive hormonal therapy and were continuously infused with 4.5%o NaCl and 5% glucose through a jugular catheter during the survival period (73-116 h). Urinary volume and Na + and K + concentrations in the urines were measured twice a day in order to adjust the infused volumes to urinary ionic excretion. The cats were turned twice a day at 0700 h and 1900 h. Thiamphenicol (250 mg) was injected i.m. daily to prevent infection.
2.4. Paradoxical sleep criteria
As described previously [15], two states were easily recognized in the polygraphic recording of a chronic pontine cat. 'Waking' state was characterized by the presence of an EMG tone, high amplitude discharges of Adrian waves in the olfactory bulb and the absence of PGO waves. 'PS' state was characterized by complete muscle atonia on the EMG, the presence of PGO wave and eye movement bursts and the absence or decrease in Adrian waves [12] (Fig. 2). The states were visually scored. The PS cycle was measured as the duration of the intervals between two successive PS episodes, provided that these episodes belonged to the same gas session (see below).
2.5. Oxygen procedure
Recordings started on the first post-operative day (D1) which was used as a control period. Oxygen 1.5-2 I/min) was then administered during the second and third postoperative days (D2 and D3) periodically for 4- or 12-h periods with a mask, for the fractional inspired 02 concentration FIO2 to be kept around 60%. In the other periods, the cats were breathing room air (Fl02 = 21%). In three cats, arterial blood gases were periodically measured through a catheter located in the femoral artery, in the following conditions: during anesthesia before surgery and on the days following surgery while breathing room air and the hyperoxic mixture. In addition, arterial blood gases were measured in awake unrestrained intact cat, while breathing room air and the hyperoxic mixture, in order to provide data on normal kPa02 for awake cat. Respiratory frequency was measured from Adrian waves in all cats.
2.6. Necropsy
At the end of the experiments, the cats were sacrificed under deep pentobarbital anesthesia (30 mg/kg i.v.). The brain was removed and fixed in 10% formol. A necropsy of major organs, including the lungs and the adrenal glands, was performed.
2.7. Statistical methods
Statistics were performed on Statgraphics 2.6 software (Manugistics, MD), using Wilcoxon test. Significance was defined at a risk < 0.05.
3. Results
3.1. Effects of hyperoxia on arterial blood gases
In the three pontine cats in which arterial blood gases were measured, C02 arterial pressure (PaC02) was not significantly different in normoxia (3.6 ± 0.8 kPa; mean ± S.D.) and in hyperoxia (3.1 ± 0.1 kPa, p = 0.62). Arterial pH was not modified (7.39 ± 0.03 in normoxia vs. 7.42 ± 0.01 in hyperoxia, p = 0.44). As expected, Pa02 increased significantly from 17.1 ± 2.6 kPa in normoxia to 58.4 ± 6.9 kPa (p < 0.001) in hyperoxia. Arterial blood gases obtained in different conditions (awake unrestrained intact cats breathing room air or under hyperoxia, anesthetized intact cat breathing room air or under hyperoxia, and pontine cat before, during and after hyperoxia) are displayed in Table 1.
3.2. Effects of hyperoxia on cardiorespiratory function
Heart rate measured on the EKG was similar in both sessions (148 ± 3 beats/min, mean ± S.E.M. in normoxia vs. 152 ± 4 in hyperoxia, p = 0.63). Respiratory frequency was not modified (20.1 ± 0.5 cycles/min in normoxia vs. 21.2 ± 0.9 in hyperoxia, p = 0.25).
3.3. Effects of hyperoxia on thermogenesis
It was possible to observe that hyperoxia increased thermogenesis, since the rate of cooling slowed down. When the incubator stopped, the time to reach minimal CT increased from 109.9 ± 3 min to 126.2 ± 5 min (p = 0.01).
3.4. Effects of hyperoxia on PS duration
On average, total PS duration cumulated over 4-h periods increased by 85% (12.4 ± 2.1 min in normoxia vs. 22.7 ± 4.8 min in hyperoxia, p = 0.03). In two cats J122, F122), PS was absent during room air breathing and appeared only after the first hyperoxic session.
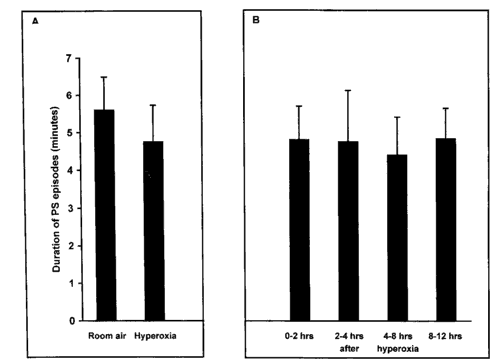
Individual duration of PS episodes was not different breathing room air and under hyperoxia (5.6 ± 0.2 min vs. 4.8 ± 0.2 min, p = 0.16). Mean PS episodes durations during air breathing and hyperoxia and after cessation of hyperoxia are shown in panels A and B of Fig. 3
3.5. Effects of hyperoxia on PS cycle
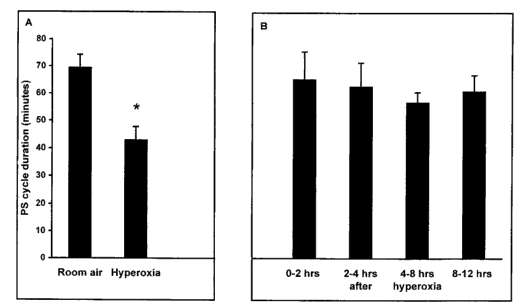
PS cycle duration dropped from 65 ± 4 min when the cats were breathing air room (n = 136) to 45 ± 4 min (n = 146, p < 0.00001) during hyperoxia. PS cycle durations for individual animals are shown in Table 2.
PS cycle duration measured during baseline room air and hyperoxia are shown on the left hand panel of Fig. 4. On the right hand panel of Fig. 4, PS cycle durations during the first 12 It following the interruption of hyperoxia are displayed. There were no significant differences between early and late measurements for the whole group.
However, when oxygen administration was interrupted, there were differences in the behavior of individual animals. In three of them (N122, C123, H124), PS cycle returned back to control values within the first 4-h period. In contrast, in the five other animals, PS cycle increased progressively and reached control values after a 4 to 12-h period. Representative examples of different behaviours are shown on Fig. 5. Cat N122 (upper trace) did not have a post-hyperoxia effect. In cat J122 (middle trace), PS was absent during air breathing and was triggered by hyperoxia, with an 8-h post-hyperoxia effect. Cat P122 (lower trace) had a 12-h post-hyperoxia effect.
4. Discussion
Our main result is that hyperoxia causes an 85% increase in PS in pontine cats, mainly through a striking increase in PS rhythm.
4.1. Thermo- and chemo-sensitive central neurons
These results cannot be explained solely by differential heat and cold sensitivities of the ponto-medullary mechanisms inhibiting or executing PS, since CT was clamped in our experiments. The increased thermogenesis during hyperoxia was limited by the temperature-clamp and, if involved in the control of PS, should have reduced and not increased PS [17].
CO2/H + -sensitive neurons have been described in the brainstem (for review, see Ref. [34]), but it is unlikely that they were involved since PC02 and pH were not modified during hyperoxic breathing. Central O2-sensitive neurons might exist [30], since hyperoxia induces hyperventilation in carotid-deafferented cats [25], and they could have been stimulated during hyperoxia. However, since we did not observe tachypnea and post-hyperventilation hypocapnia during hyperoxia, which are indirect signs of stimulation of these putative 02-sensitive neurons [5], it is unlikely that they played a significant role although minute ventilation was not measured in our preparation.
Hyperoxia may restore the animal to a normal level required for normal neuronal function. Since arterial oxygenation was similar in pontine preparation while breathing room air and in normal awake and anesthetized animal, hyperoxia seems to restore supranormal level in pontine preparations.
4.2. Discrepancy between hypothermia and hyperoxiamediated effects
These experiments were set up in order to explain the linear increase in PS observed with decreasing CT [17,18]. However, if hyperoxia and hypothermia both increase PS, they have slightly different effects. Hypothermia increases the duration of PS episodes without modifying PS rhythm [19], whereas hyperoxia increases PS rhythm without modifying the duration of PS episodes. However, the effects of hypothermia cannot be reduced to a better cerebral 02 supply adequacy. Hypothermia modifies the velocity of enzymatic reactions (for review, see Ref. [4] and therefore could affect PS control through mechanisms different from those implied in hyperoxia. However, PS cycles observed during hyperoxia in pontine cats are almost twice longer than the PS cycles of intact cats [8], meaning that suprapontine mechanisms influence a putative pontomedullary 'PS-clock'. The effect of hyperoxia on PS rhythm is similar to what is observed in those pontine preparations where a small island containing the hypothalamus and the hypophysis is left in place [19]. Such preparations kept at a 34°C CT exhibit PS with a cycle of 47 ± 4 min (J.-P. Sastre, personal communication), a measure close to the 45 ± 4 min observed in our hyperoxic pontine cats. Hyperprolactinernia is thought to be responsible for PS rhythm increase in 'small island' preparations [20]. Prolactine and 02 share the ability to stimulate pyruvate dehydrogenase, an enzyme of oxidative metabolism [38]. The putative mechanism of interaction between this enzymatic activity and PS is discussed hereafter.
An exciting finding in our study was the prolongation of the increase in PS rhythm observed after cessation of hyperoxia in five animals, despite an immediate normalization of arterial blood gases. This long-lasting effect suggests that the putative 'PS-clock' may accumulate an 02-dependent substrate, released for 4 to 12 h after the end of hyperoxia.
4.3. Cholinergic PS systems and oxidative metabolism
In two cats who failed to exhibit PS in control conditions, hyperoxia was sufficient to gate PS, while in the other cats it modified the PS-clock, making it run twice faster. Therefore, it is likely that PS depends upon the oxidative metabolism. It is now acknowledged that PS involves cholinergic structures located in the pons-medulla (for review, see Ref. [14]). Acetylcholine (ACh) synthesis is regulated by pyruvate dehydrogenase (PDH), an enzyme that converts pyruvate into acetylcoenzyme A (AcCoA) via the Krebs cycle. AcCoA is both a precursor moiety of ACh and a substrate for oxidative phosphorylation in the mitochondria. ACh synthesis is critically dependent on AcCoA supply [10]. PDH is activated by a phosphatase and inactivated by a kinase and there is evidence both in vitro and in vivo that the activation of PDH is dependent upon the mitochondrial redox state [38]. For this reason, mild hypoxia alters ACh biosynthesis [6,29]. This might explain why PS is selectively suppressed by hypoxia, whereas slow-wave sleep may be normal or increased in normal cats [3,13]. On the other hand, waking mechanisms may utilize the conversion of pyruvate into lactate through lactate dehydrogenase (LDH), since there may exist an uncoupling between glucose and 02 consumptions during active wake as shown by recent positron emission tomography investigations [9]. It is worth noticing that the structures that inhibit PS, for instance the locus coeruleus, are rich in LDH [37], whereas ACh-containing neurons of the pons-medulla are rich in PDH [26,33].
4.4. Model of interaction between oxidative metabolism, temperature and sleep-wake cycle
These data led us to suggest the following hypothesis concerning the striking dependency of PS upon CT in pontine cats. Above the threshold of 36°C, there seems to exist some uncoupling between glucose and 02 consumptions. In these conditions, the metabolism of pyruvate would be channelled mostly towards the LDH pathway which could be activated within the monoaminergic systems. The relative deficit of oxidative metabolism would not permit PDH to be activated and brainstem ACh synthesis would be impaired. This would explain the permanent waking observed above 36°C. Below this threshold, there is, in the absence of any regulatory hypothalamic mechanisms, a 10% decrease in 02 consumption at 34°C [7]. Since 02 supply does not decrease at the same rate as 02 consumption [11,31], a condition permitting the oxidative metabolism of pyruvate into AcCoA, ACh will appear and allow the appearance of PS with a circahoral rhythm. Hyperoxia will increase the activation of PDH phosphatase and the synthesis of ACh, which could explain the 30-50% increase in the ultradian rhythm of PS.
In conclusion, cholinergic mechanisms of PS might be critically dependent upon cerebral 02 supply which controls the oxidative metabolism of pyruvate in the pontine cats. The confirmation of this hypothesis would require the recording of cerebral 0, supply, blood flow and tissue lactate in pontine preparation.
Acknowledgements
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM U480) and by the Centre National de la Recherche Scientifique (CNRS URA 1195) and performed in the Department of Experimental Medicine, Claude Bernard University, Lyon, France. We are grateful for the assistance of G. Debilly with statistical analysis and J.-Ph. Derenne and Catherine Limoge for manuscript correction.
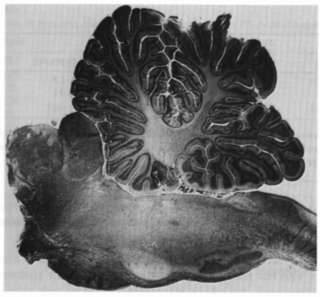
Fig. 1. Sagittal view of a pontine cat
 |
|
The brain is sectioned at the ponto-mesenphalic
junction (coronal section in A2.5 stereotaxic plane) and cerebral
structures rostral to the section are removed. Isolated olfactive
bulbs are left in place (not shown in the figure)
|
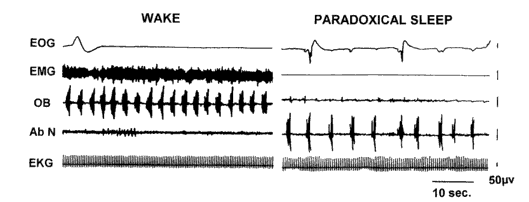
Fig. 2. Polygraphic recording of a pontine cat
 |
|
Left panel: "waking" state; right
panel: 'paradoxical sleep' state. EOG: electrooculogram; EMG:
neck electromyogram; 0B: olfactory bulb activity; Ab N: abducens
nucleus activity; EKG: electrocardiogram. Waking state is characterized
by high amplitude discharges of Adrian waves in 0B, while PS is
characterized by ponto-geniculo-occipital burst activity in the
abducens nucleus, rapid eye movements, complete muscular atonia,
and absence of Adrian waves.
|
Fig. 3 Effect of hyperoxia on PS episodes duration
 |
|
There were no significant differences between
mean PS episodes duration when breathing room air and under hyperoxia
(panel A) and after cessation of hyperoxia (panel B). Bars indicate
S.E.M.
|
Fig. 4. Effect of hyperoxia on PS cycle duration
 |
|
Mean PS cycle is reduced by one-third when breathing
the hyperoxic mixture (panel A). When oxygen administration is
interrupted, PS cycle return back to control values either within
4 h, or progressively after a 4- to 12-h period (panel B). Bars
indicate S.E.M.
|
Fig. 5 Hypnograms of three pontine cats
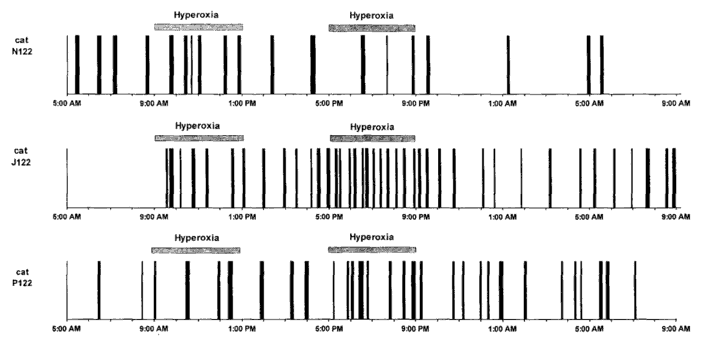 |
|
Vertical bars represent PS (paradoxical sleep)
episodes varying in duration. Thick shaded horizontal bars indicate
hyperoxic breathing, In cat N122 (upper trace), hyperoxia caused
an increased PS frequency, returning back to baseline values when
breathing room air, In cat J122 (middle trace), PS was absent
at the beginning of the study during air breathing and was apparently
triggered by hyperoxia; this increased frequency persisted for
8 h after cessation of hyperoxia (post-hyperoxia effect). In cat
P122 (lower trace), PS was present during air breathing, its frequency
increased during hyperoxia and remained elevated 12 h after cessation
of hyperoxia. On average, individual PS durations were similar
in the various conditions tested.
|
Table 1 : Blood gases in intact and pontine cat
| Intact awake cat (37°C) | Intact anesthetized cat (37°C) | Pontine cat (34°C) | |||||
| Room air | Hyperoxia | Room air | Hyperoxia | Room air | Hyperoxia | Post-hyperoxia | |
| P02 | 16.4 ± 2.6 | 45.5 ± 3.6 | 14.8 ± 0.5 | 36.8 ± 9.8 | 17.1 ± 2.6 | 58.4 ± 6.9* | 19.9 ± 1.5 |
| PC02 | 3.7 ± 0.1 | 4.1 ± 0.5 | 3.2 ± 0.3 | 3.1 ± 0.2 | 3.6 ± 0.8 | 3.1 ± 0.1 | 2.7 ± 0.1 |
| pH | 7.41± 0.05 | 7.37 ± 0.04 | 7.41 ± 0.04 | 7.45 ± 0.03 | 7.39 ± 0.03 | 7.42 ± 0.01 | 7.44 ± 0.01 |
|
Arterial blood gases (mean ± standard deviation, in kPa) were measured in intact awake cat and in three cats, during anesthesia before surgery and on the days following surgery, while breathing room air and the hyperoxic mixture. Central temperature is indicated in brackets. Asterisks indicate a significant difference (p < 0.05) in P02 measured during hyperoxia and while breathing room air. |
|||||||
Table 2 : Effect of breathing room air or hyperoxic mixture on PS cycle duration
| Animal | Room air | Hyperoxia |
| C122 | 46 ± 2 | 29 ± 2 |
| C123 | 89 ± 14 | 69 ± 12 |
| F122 | 141 ± 28 | 123 ± 16 |
| H124 | 101 ± 15 | 113 ± 27 |
| J122 | 52 ± 5* | 22 ± 2 |
| N122 | 56 ± 28 | 51 ± 9 |
| P122 | 71 ± 11 | 48 ± 8 |
| Z123 | 63 ± 6 | 51 ± 5 |
| Mean | 65 ± 4 | 45 ± 4 |
| Mean PS cycle duration is indicated in minutes ± standard error. The two cats with an asterisk had no PS at baseline: these data were obtained during the experimental days after PS appearance. | ||
The electrical activity of the mammalian olfactory bulb Electroenceph. Clin. Neurophysiol. 2 (1950) 377-388.
[2] A. Asami, T. Hori, T. Kiyohara, T. Nakashima
Convergence of thermal signals on the reticulospinal neurons in the midbrain, pons and medulla oblongata Brain Res. Bull. 20 (1988) 581-596.
[3] T.L. Baker, D.J. McGinty
Sleep-waking patterns in hypoxic kitten Dev. Psychobiol. 12 (6) (1979) 561-575.
[4] C. Balny
Temperature and enzyme function in: A. Malan, B. Canguilhem (Eds.), Living in the Cold, Colloque Inserm/John Libbey Eurotext, London, 1989, pp. 157-166.
[5] H. Becker, 0. Poli, S.G. McNamara, M. Berthon-Jones, C.E. Sullivan
Ventilatory response to isocapnic hyperoxia J. Appl. Physiol. 78 (2) (1995) 696-701.
[6] J.P. Blass, G.E. Gibson
Consequences of mild, graded hypoxia Adv. Neurol. 26 (1979) 229-253.
[7] D.W. Busija, CW. Leffler
Hypothermia reduces cerebral metabolic rate and cerebral blood flow in newborn pigs Am. J. Physiol. 253 (1987) 869-873.
[8] F. Delorme, P. Vimont, D. Jouvet
Etude statistique du cycle veille sommeil chez le chat C. R. Soc. Biol. 158 (1964) 2128-2130.
[9] P.T. Fox, M.E. Raichle, M.A. Mintun, C. Dence
Non-oxidative glucose consumption during focal physiologic neural activity Science 241 (1988) 462-464.
[10] G.E. Gibson, M. Shimada
Studies on the metabolic pathway of acetyl group for acetylcholine synthesis Biochem. Pharmacol. 29 (1980) 167-174.
[11] F. Gollan, J.E. Hoffman, R.M. Jones
Maintenance of life of dogs below 10°C without hemoglobin Am. J. Physiol. 179 (1954) 640646.
[12] P. Homeyer, J.P. Sastre, C. Buda, M. Jouvet>
Suppression of Ottoson waves in the isolated olfactory bulb during sleep in the pontine cat NeuroReport 6 (1995) 773-776.
[13] J. Huertas, J.K. McMillin
Paradoxical sleep: effect of low partial pressure of atmospheric oxygen Science 159 (1968) 745-746.
[14] B.E. Jones
Paradoxical sleep and its chemical/structural substrates in the brain Neuroscience 40-3 (1991) 637-656.
[15] M. Jouvet
Recherches sur les structures nerveuses et les mécanismes responsables des différentes phases de sommeil physiologique Arch. Ital. Biol. 100 (1962) 125-206. FULL TEXT
[16] M. Jouvet
The role of monoamine and acetylcholine-containing neurons in the regulation of the sleep-waking cycle Ergeb. Physiol. 64 (1972) 166-307.
[17] M. Jouvet, C. Buda, J.P. Sastre
Increase of paradoxical sleep during hypothermia in pontine cats in: J. Home (Ed.), Sleep '88, Gustav Fisher Verlag, Stuttgart, 1989, pp. 85-90.
[18] M. Jouvet, C. Buda, J.P. Sastre
Hypothermia induces a quasi permanent paradoxical sleep state in pontine cats in: A. Malan, B. Canguilhem (Eds.), Living in the Cold, Colloque, Inserm/John Libbey Eurotext, London, 1989, pp. 487-497.
[19] M. Jouvet, C. Buda, I. Arnulf, J.P. Sastre
Evolution of the ultradian rhythm of paradoxical sleep in pontine cats during hypothermia Sleep Res. 20A (1991) 579.
[20] M. Jouvet, C. Buda, J.P. Sastre
Existe-t-il un pacemaker bulbaire responsable du rythme ultradien du sommeil paradoxal? Arch. Ital. Biol. 134 (1995) 39-56.
[21] Karacan, S.M. Wolff, R.L. Williams, C.J. Hursch, W.B. Webb The effects of fever on sleep and dream patterns Psychosomatics 9 (1968) 331-339.
[22] S. Kent, M. Price, E. Satinoff<
Fever alters characteristics of sleep in rats Physiol. Behav. 44-6 (1988) 709-715.
[23] J. Laszy, A. Sarkadi
Hypoxia-induced sleep disturbance in rats Sleep 13-3 (1990) 205-217.
[24] K. Masek, 0. Kadlecova, H. Raskova
Brain amines in fever and sleep cycle changes caused by streptococcal mucopeptide Neuropharmacology 12 (1973) 1039-1047.
[25] M.J. Miller, S.M. Tenney
Hyperoxic hyperventilation in carotid deafferented cats Respir. Physiol. 23 (1975) 23-30.
[26] T.A. Milner, C. Aoki, K.-F.R. Sheu, J.P. Blass, V.M. Picket
Light microscopic immunocytochernical localization of pyruvate dehydrogenase complex in rat brain: topographical distribution and relation to cholinergic and catecholarninergic nuclei J. Neurosci. 7-10 (1987) 3171-3190.
[27] D.A. Morilak, C.A. Fomal, B.L. Jacobs Effects of physiological manipulations on locus coeruleus neuronal activity in freely moving cats Brain Res. 422 (1987) 17-23.
[28] J.R. Pappenheimer Sleep and respiration of rats during hypoxia J. Physiol. (London) 266 (1971) 191-207,
[29] I.R. Park, M.B. Thom, H.S. Bachelard
Threshold requirements for oxygen in the release of acetylcholine from, and in the maintenance of the energy state in, rat brain synaptosomes J. Neurochem. 49 (1987) 781-788.
[30] D.W. Richter, A. Bishoff, K. Anders, M. Bellingham, U. Windhorst
Response of the medullary respiratory network of the cat to hypoxia J. Physiol. 443 (1991) 231-256.
[31] T. Sakamoto, W.W. Monafo
Regional blood flow in the brain and spinal cord of hypothermic rats Am. J. Physiol. 257 (1989) 785-790, (Heart Circ. Physiol. 26).
[32] B.K. Siesjö
Brain energy metabolism in: Wiley (Ed.), New York, 1978, pp. 1-607.
[33] S.H. Sterri, F. Fonnum Acetyl-CoA synthesizing enzymes in cholinergic nerve terminals J. Neurochem. 35-1 (1980) 249-254.
[34] S. Suguma, N. Shimokawa, J. Okada, M. Miura In vitro study of H ± sensitive neurons in the ventral medullary surface of the neonate rats Brain Res. 777 (1997) 95-102.
[35] H. Swan
Thermoregulation and bioenergetics patterns for vertebrate survival American Elsevier, New York, 1974, 414 pp.
[36] L.A. Toth, J.M. Krueger Somnogenic, pyrogenic, and hematologic effects of exper
imental pasteurellosis in rabbits Am. J. Physiol. 258 (1990) 536-542.
[37] I.W. Borowski, R.C. Collins
Metabolic anatomy of brain: a comparison of regional capillary density, glucose metabolism, and enzyme activities J. Comp. Neurol. 288 (1989) 401-403.
[38] O.H. Wieland
The mammalian pyruvate dehydrogenase complex: structure and function Rev. Physiol. Biochem. Pharmacol. 96 (1983) 123-170.