| Biogenic Amines and the States of Sleep |
| Michel Jouvet Science 163 (862) pages : 32-41 (1969) |
Introduction
Pharmacological and neurophysiological studies suggest a relationship between brain serotonin and sleep.
In the past 10 years the study of sleep mechanisms has developed very rapidly and has become one of the major fields of neurophysiological and psychological research. This rapid growth is in part due to some discoveries which have changed the classical neurophysiological picture of sleep from the concept of a passive resting state of the brain to that of a heterogeneous and complicated suc cession of active phenomena.
Very seldom in the history of physiology has so much effort been devoted to the description, quantification, classification, and delimitation of such a complex phenomenon of almost totally un known function. For this reason, I find it unnecessary to present a detailed review of the phenomenology of sleep and attempt instead to discuss the mechanisms involved in sleep.
I first outline the four major concepts which have changed the classical theory of sleep. Second, I describe the neuropharmacological and histochemical data which have made possible the link between structural data and functional mechanisms. Finally, I present some recent findings which have led to the belief that a complex of monoamine-containing neurons are of para mount importance in the process under lying the succession of sleep states.
The Four Major Concepts
Sleep, an active phenomenon. Until recently neurophysiologists had considered sleep to be a unique passive state opposed to waking. With the discovery of the ascending reticular activating system (1), waking could be explained in terms of an increase in the activity of this activating system, and sleep, in terms of the passive dampening of the system (2). This theory requires only one system to explain the succession from waking to sleep and thus obeys the law of economy according to which a living organism always selects the simplest possible means of realizing a potential function. This theory of sleep was widely accepted initially but has since been drastically challenged.
The finding that sleep could be induced by electrical stimulation of different parts of the brain was at variance with the passive theory of sleep. How ever the "passive theorists" could easily defend their position by questioning the validity of electrical stimulation. It is exceedingly difficult to conceive of a system of sleep as diffuse as that suggested by studies of stimulation; sleep can be induced by stimulation of many different parts of the brain, including those which overlap the ascending reticular activating system: cortex, thalamus, subthalamus, hypothalamus, mesencephalon, pons, cerebellum, medulla, spinal cord, sensory nerves, and so on (3). In the absence of stimulation a cat with electrodes permanently implanted in its brain may sleep "spontaneously" 60 to 70 percent of the time (4). Thus the probability that the cat will "fall asleep" spontaneously after electrical stimula tion of the brain is greater than the chance level; very few experiments deal ing with "hypnogenic" stimulation (electrical or chemical) provided conclusive and statistically significant data. Finally it was realized that sleep could be induced only by stimulation of low frequency corresponding to the frequency of the sleep spindles. In sum, the finding that sleep could be induced by stimulation was not a major proof that the passive theory of sleep was invalid.
To prove the existence of an active sleep-inducing system it was necessary to produce a state of insomnia by means of a lesion destroying this system. The first step in this direction was transection of the brain stem immediately be low the ascending reticular activating system. A mid-pontine transection was shown to produce a definite increase in waking behavior and in electroencephalographic traces characteristic of waking (5). This suggested that synchronizing or hypnogenic structures were located in the lower brain stem. However, limited lesions in many structures of the pons or the medulla (mostly in the lateral part of the lower brain stem) did not reveal a definite location of these hypnogenic structures (6). Nevertheless, in 1960 most neurophysiologists were ready to accept the hypothesis that there are sleep-inducing mechanisms which can actively dampen the activity of the ascending reticular activating system.
This concept was more readily accepted when, at about the same time, new and conclusive data indicated that sleep could not be considered a unique phenomenon opposed to waking but did in fact, at least in the mammalian and avian brain, consist of two successive states.
Sleep or sleeps. The concept of the dichotomy of sleep has very ancient roots, predating even modern physiology (7). Major advances are marked by publications that appeared in the late 1950's (8). Since this topic has been the subject of many recent reviews (9), I shall survey it very rapidly.
In brief, the mammalian sleeping brain successively passes through two states which can be recognized very easily through the application of poly graphic techniques in the study of ani mals with electrodes permanently im planted in the brain ( Fig. 1).
The first state has been called slow wave sleep (10). In this state the animal has a posture characteristic of sleep, its eyes are closed, and the pupils are myotic. A degree of postural tonus al ways remains in some muscle groups of the body (including those of the neck). The electrical activity of the cortex is characterized by spindles and slow waves.
After a time this state is succeeded by a totally different state. I have given it the name of paradoxical sleep (11) because I found that the association of a cortical activity similar to that of waking with a total absence of electromyo graphic activity associated with muscular activity of the neck appeared paradoxical. Paradoxical sleep has two different phenomenological aspects, which may be described briefly as follows.
1 ) Tonic activity. There is a fast, low-voltage cortical activity similar to that of waking, associated with a regular theta rhythm in the hippocampus and a total disappearance of electromyographic evidence of muscular activity. Tonic phenomena persist for several minutes and are accompanied by phasic behavioral and electrical phenomena which are highly characteristic of paradoxical sleep in most mammals.
2) Phasic activity. Rapid eye movements (50 to 60 per minute) occur in a rather stereotyped pattern which is different from that of waking. They are associated with a cortical and subcortical activity which has been termed pontogeniculo-occipItal. activity, or deep sleep waves (12, 13). Such phasic activity is composed of high-voltage waves which can be recorded from the reticular formation of the pons, from the lateral geniculate, and from the occipItal. cortex. They are almost identical to those recorded during visual attention (13). Apparently these "PGO" waves occur in a rather fixed pattern, since they occur at a fairly constant daily rate in the cat (14,000 + 3000 waves per day) (14). They occur transiently during slow-wave sleep, and they always precede paradoxical sleep by some 30 to 45 seconds. Their rate during paradoxical sleep is also fairly constant (0 + 5 waves per minute). The inner most mechanism of these PGO waves is a fascinating problem which has not yet been solved, despite many investigations (12, 13, 15). During paradoxical sleep a "spontaneous" activity occurs periodically which resembles the electrical activity of visual input during waking. Neurophysiological evidence suggests that, during this state of sleep, active phenomena are triggering electrical events (PGO waves) and behavioral events (rapid eye movements) which may have earlier counterparts in the waking events of visual input. This suggestion may provide a cue for understanding the function of paradoxical sleep.
This brief description of the phenomenological events occurring during slow wave sleep and paradoxical sleep is certainly not sufficient to prove that these two types of sleep are associated with two different functional states of the brain. However, this association has heen well demonstrated by the finding that paradoxical sleep may be selectively suppressed without altering slow-wave sleep, through the use of various drugs and through specific lesions. These neuropharmacological and neurophysiological data favoring a dualistic theory of sleep are strongly supported by ontogenic and phylogenic studies.
Numerous investigations (16) have shown that, immediately after birth, most newborn mammals of species whose central nervous system is incompletely developed at birth (for example, the cat, rat, and rabbit) manifest only the succession from waking to paradoxical sleep. Slow-wave sleep appears later, when the maturation of the cortical network is achieved. In newborn mammals of species whose central nervous system is well developed at birth (for example, the guinea pig and lamb), slow-wave sleep and paradoxical sleep alternate in a periodic fashion immediately after birth.
Even if the phylogenetic story of sleep is far from complete, the dichotomy between slow-wave sleep and paradoxical sleep has been well demonstrated. Paradoxical sleep has been shown to be absent in fish (17), probably absent in reptiles (18), present but very short-l;ved in birds (19), and present in every mammalian form studied up to now, from the opposum (20) to the elephant (21) and, of course, man (8).
In summary, in phenomenological, neuropharmacological, neurophysiological, ontogenic, and phylogenic studies, two qualitatively different states of sleep involving two different mechanisms and probably serving two different functions are distinguishable.
Quantitative aspect of the sleep states. The first step in the study of the sleep states was description of their qualitative patterns. During this time no quantitative study of sleep was attempted, due especially to economic and technical difficulties. Recent data, however, have shown that the sleep states are, like the rectal temperature, the heart rate, and the basic metabolism, a biological constant (4). It is thus possible to consider slow-wave sleep and paradoxical sleep as a quantitative index of the innermost mechanisms of the brain. This advance has made it possible to study the sleep states in relation to quantitative alterations in brain func tion such as result from drug injection or limited brain lesion, or in relation to data obtained through biochemical analysis. It is also possible to correlate the circadian variation of the sleep states with the circadian biochemical variation in the brain (22).
Sleep is a subject of "wet" neurophysiology.
With the advent of a growing interest in the biochemistry of the nervous system, F.O. Schmidt has introduced the terms "dry" and "wet" neurophysiology, with reference, respectively, to the electrical and the neurohumoral phenomena (23). Ten years ago the passive theory of sleep could be relatively easily explained in terms of dry-neurophysiology mechanisms (for example, the reduction of afferent input to the ascending reticular activating system, neuronal fatigue, and so on) (2). When it was realized that sleep is a diffuse system, a dry-neurophysiology mechanism could no longer explain the circadian variations of the organism. The time constant of the electrical potentials of the brain is of the order of milliseconds and can therefore not be that of the circadian or ultradian rhythmicity of the sleep states. This concept is most conclusively demonstrated by the alteration in sleep states that is effected by selective deprivation of paradoxical sleep (24). Following such deprivation, a long-lasting , rebound of paradoxical sleep (increases in frequency of phase and frequency of PGO waves) (14) occurs, which usually lasts for a period approximately half the duration of the deprivation. No dry neurophysiology mechanism, however sophisticated, can explain this rebound phenomenon, which may last for several days or even weeks.
Thus any theory of sleep must depend upon the concept of wet neurophysiology for its validity.
In summary, recent major advances in the neurophysiology of sleep have led to the following concepts.
1) Sleep is an active state of the brain. Thus, it should be possible to produce insomnia through circumscribed lesion of the brain.
2) Sleep is not a single phenomenon but, instead, consists of two different states which involve two different mech anisms of the brain. Thus it should be possible to selectively suppress either slow-wave sleep or paradoxical sleep by limited lesion or by drugs.
3) The sleep states can be quantita tively measured. Thus it should be possible to correlate their quantity with any suitable quantitative biochemical, neuropharmacological, or structural alteration.
4) Biochemical mechanisms which cannot be explained exclusively in terms of short-term neuronal mechanism are involved in sleep states.
| Group | Number in Group | Amount of Slow Wave Sleep (%) | Amount of Paradoxical Sleep (%) | % of raphe Intact | P* | % of cerebral sertonin | P* | % of cerebral noradrenalin | P* |
| A | 12 | 48.5+-7.5 | 9.5+-2.5 | 95.4+-12 | - | 90+-23 | - | 102+-17 | |
| B | 6 | 30+-2.7 | 5.5+-2.7 | 77.5+-19 | .02 | 68+-18 | .10 | 99+-22 | NS |
| C | 10 | 16.5+-2.2 | 1+-0.7 | 64+-8.5 | .001 | 54+-22 | .01 | 93+-17 | NS |
| D | 6 | 9+-1.5 | 0 | 35+-10 | .001 | 29+-11 | .001 | 92.5+-17 | NS |
* Student's t-test values for P obtained by comparison with group A; NS; not significant. Each P column refers to the column that immediately precedes it.
Table 1. Comparison, for various groups of cats with brain lesions, of (i) the amount of sleep following surgery, expressed as a percentage of total recording time (10 to 13 days); (ii) the percentage of the raphe system left intact; and (iii) the amounts of serotonin and noradrenalin in the brain rostral to the lesion, expressed as percentages of the amounts in the brains of normal control cats. The group divisions are based on the extent of the lesion and the amount of sleep following surgery: (A) cats with insignificant destruction of the raphe system-an amount having no effect on sleep; (B and C) cats with major but less than total destruction of the raphe system; (D) cats with almost total destruction of the raphe system. The percentages are mean values for an entire group, plus standard deviation.
Biogenic Amines and the Sleep States
Due to a lack of adequate techniques, it is only very recently that a neuro pharmacological study of sleep has been successfully carried out. It was not until the technique of making continuous long-term electroencephalographic recordings had been perfected and suitable pharmacological techniques had been developed that this field was adequately explored. The fact that the monoamines themselves (serotonin and noradrenalin) do not cross the mammalian blood brain barrier presented a major technical difflculty. In birds, whose blood brain barrier is permeable, intravenous injection of serotonin or noradrenalin leads to slow-wave sleep, as indicated by hehavior and by electroencephalographic recording (25). Attempts to by pass this barrier in mammals through local application or through injection of serotonin into the brain have led, also, to an increase of cortical synchronization in short-term experiments (26). Injection into the ventricles has given questionable results (27). The use of precursors of these amines which readily cross the blood-brain barrier has been a step forward. The increase of cerebral serotonin (through intravenous or intraperitoneal injection of its precursor 5-hydroxytryptophan (5 HTP)) leads first to a state which resembles slow-wave sleep (28). However, this drug leads to a suppression of paradoxical sleep for 5 to 6 hours; the suppression is followed by a secondary rebound (29) The injection of dihydroxyphenylalanine (DOPA), which is a precursor of both dopamine (DA) and noradrenalin, produces an increase in waking (30, 31), whereas the injection of dihydroxyphenylserine, which is be lieved to be a direct precursor of noradrenalin (32), increases slow-wave sleep and paradoxical sleep (33). More recently it has been demonstrated that numerous drugs known to act on the concentrations of monoamines in the brain act in a rather predictable way on the sleep states. Producing a decrease in serotonin and noradrenalin, reserpine (0.5 milligram per kilogram in the cat), although inducing a "state of tranquility" (34), suppresses the appearance of slow-wave sleep for 12 hours and of paradoxical sleep for about 24 hours, whereas it triggers continuous PGO waves similar to those of paradoxical sleep. Secondary injection of 5-hydroxytryptophan, which restores a normal brain concentration of serotonin, results in the immediate reappearance of electroencephalographic patterns of slow wave sleep, whereas the injection of di hydroxyphenylalanine, which restores the catecholamine concentration following the administration of reserpine, leads to the reappearance of paradoxical sleep. Thus there was an implication that serotonin may be involved in slow wave sleep whereas the catecholamines rnay be involved in paradoxical sleep (30, 35). Monoamine oxidase (MAO) inhibitors (nialamide, iproniazid, phenylisopropylhydrazide), which act upon both brain monoamines by inhibiting their catalolism and by thus increasing their concentration in the brain, were shown to act dramatically upon sleep states. Most of the monoamine oxidase inhibitors utilized have a very specific suppressive effect upon paradoxical sleep and increase slow-wave sleep in the cat. This suppressive effect is so intense that it is even operative when the "need" for paradoxical sleep is greatly enhanced following paradoxical sleep deprivation (30, 36). This suggests that monoamine oxidase is necessary to the passage from slow-wave sleep to paradoxical sleep. These findings were of only limited significance in view of the enormous complexity of the biochemical mechanism of the brain. Most of these drugs were acting upon both indoleamines and catecholamines and therefore might very well be inter fering with the cyclic alteration of the brain's electrical activity.
More recently, however, some drugs that act selectively upon serotonin or catecholamines have been discovered. The most interesting ones for the neurophysiologist are those which inhibit the synthesis of monoamines (p-chlorophenylalanine for serotonin; alpha-methylparatyrosine for dopamine and noradrenalin, or disulfiram for noradrenalin). It is thus possible to alter selectively the metabolism of one monoamine in the study of the sleep states.
Other major steps came at this time, which permitted a more rigorous way of thinking by closing the gap between neuropharmacology, neuroanatomy, and neurophysiology. Indeed, histofluorescence techniques have made possible the precise topographical study of nerve cells containing monoamines (37). It was demonstrated that the serotonin containing neurons were located mostly in the raphe system (37, 38), the noradrenalin-containing neurons in the lateral part of the bulbopontine tegmentum (principally in the locus coeruleus), and the dopamine-containing neurons in the ventral part of the mesencephalon (37).
It was shown that the cell bodies of the monoamine-containing neurons send terminals to widespread regions of the spinal cord and brain, of which the ascending pathways, either mono- or polysynaptic, follow the medial forebrain bundle (37, 39). Moreover, it was shown that sectioning of the axons would suppress specific fluorescence of the corresponding terminals after 8 to 10 days (37). This finding made it possible to attack specific groups of monoamine containing neurons by classical neurophysiological techniques and to correlate such destruction with biochemical analysis, a critical period of time being allowed between destruction of the nerve cells and death of the animal (40).
Thus it was possible to alter the concentrations of monoamines in the brain either by inhibiting their synthesis or by destroying monoamine-containing nerve cells.
Insomnia Following Selective Decrease of Cerebral Serotonin
Pharmacologically induced decrease. It has been shown that p-chlorophenylalanine selectively decreases the concentration of serotonin in the brain without altering the concentration of either noradrenalin or dopamine. This selective action is probably effected by inhibition of the enzyme tryptophan hydroxylase (41). In cats (42, 43), rats (44), and monkeys (45), the action of this drug has been recently studied by means of electrodes permanently implanted in the brain. After a single injection of p-chlorophenylalanine (400 milligrams per kilogram) in the cat (42) no apparent variation of behavior or of the electroencephalographic record is observed during the first 24 hours. This fact demonstrates that the drug in itself has no direct pharmacological action upon the brain. Following this period there is an abrupt decrease in the in cidence of both sleep states, and after 30 hours there is almost total insomnia, as shown by continuous quiet waking behavior, mild mydriasis, and a permanent rapid low-voltage cortical activity. The reoccurrence of sleep begins after the 60th hour and is accompanied by the appearance of continuous phasic waves in the lateral geniculate body and occipItal. cortex (similar to the PGO wave activity usually observed during the slow-wave sleep that immediately precedes paradoxical sleep and during paradoxical sleep). Discrete episodes of paradoxical sleep occur, following short periods of slow-wave sleep and even immediately following waking. Slow-wave-sleep episodes of longer duration gradually reappear at shorter intervals. Normal patterns of sleep reoccur after about 200 hours. A significant correlation has been found to exist between the decrease in slow-wave sleep and the decrease in cerebral concentrations of serotonin (at the level of serotonin-containing terminals in the rat). There was no significant alteration in the cerebral concentrations of noradrenalin or dopa mine (44) (Fig. 2).
Since p-chlorophenylalanine inhibits only the first step in the synthesis of serotonin, it is possible to bypass its blocking action and thereby reestablish the concentration of serotonin by injecting the direct precursor of serotonin. 'This has been accomplished by the injection of 5-hydroxytryptophan (which readily crosses the blood-brain barrier) following the injection of p-chlorophenylalanine. In this way it has been shown that the sleep state of the animal can be readily manipulated (42). A single injection (intravenous or peritoneal) of a very small dose of 5-hydroxytryptophan (2 to 5 milligrams per kilogram) administered when the decrease in sleep has reached its maximum (30 hours following injection of p chlorophenylalanine) results in restoration of a normal pattern of both states of sleep (Fig. 3). This normal pattern may last for a period of 6 to 10 hours, after which there is a rapid return to insomnia. In another series of experiments a control cat receiving daily doses of p-chlorophenylalanine experiences a severe and long-lasting insomnia as sociated with behavioral disturbances (anorexia, inability to walk), whereas an animal receiving balanced doses of 5-hydroxytryptophan and p-chlorophenylalanine sleeps a normal amount, or even more than normal, for several days, with no behavioral disturbances. These experiments show rather conclusively that one can manipulate sleep mechanisms at will merely by interfering with the synthesis of serotonin.
The results of this study (which are in agreement with the previous findings that serotonin injected after the injection of reserpine restores slow-wave sleep) led to the hypothesis that slow-wave sleep requires the presence of serotonin at the terminals of serotonin-containing neurons. Since other pharmacological data demonstrate that paradoxical sleep is eliminated for a long period by in jection of monoamine oxidase inhibitors (36), it is suggested that a still unknown deaminated metabolite of serotonin may be responsible for the triggering of para doxical sleep (30). The results of this pharmacological study must be critically evaluated and cannot be accepted as conclusive evidence for the existence of a serotonergic mechanism of slow-wave sleep. It must be recognized that not only the metabolism of serotonin in the brain but also that in the total periphery is altered by p-chlorophenylalanine. It is therefore difflcult to rule out a possible extracerebral factor in the dramatic insomnia which follows injection of this drug.
For this reason it is necessary to make a neurophysiological study of the role of serotonin as well as a pharmacological study. Because of the topographical localization of the serotonin-containing neurons, it was found possible to selectively destroy most of these neurons and to thus diminish the level of serotonin in the brain.
Decrease by neurophysiological techniques. By means of stereotaxic methods, the serotonin-containing neurons of the raphe system were destroyed study must be critically evaluated and in cats having electrodes permanently implanted in the brain (Fig. 4). The animals' brain waves were continuously recorded for 10 to 13 days after the operation, this being the critical period for the voiding of the serotonin-containing terminals (37). At the end of this period the cats were killed (at a fixed hour so as to avoid differences due to circadian alteration of serotonin concentration) for histological evaluation of the lesioned area of the brain and for biochemical analysis of the intact regions of the brain (telencephalon, diencephalon, mesencephalon, and spinal cord). By this method, the following information was obtained: a valid quantitative measure of the sleep states (obtained from the mean percentages of slow-wave sleep and paradoxical sleep during the 10 to 13 days of recording); a measure of the extent of the lesion (the percentage of the total raphe system destroyed), obtained by topographical analysis; and an estimate of the percentages of serotonin and noradrenalin in the brains of cats with brain lesions relative to the percentages in the brains of cats which had undergone sham operations (46).
After destruction of a large part (80 to 90 percent) of the raphe system, a continuous state of insomnia, as evidenced by the cat's behavior and by the encephalographic record, is observed during the first 3 to 4 days. In the period that follows, some slow-wave sleep is observed, but the total duration of the periods of slow-wave sleep never exceeds 10 percent of the recording time. Paradoxical sleep is never observed in these cats. Partial lesions of the raphe system (in the anterior or posterior regions) result in an insomnia which is less pronounced (due to a gradual recuperation after the first 2 days). In cats with such lesions paradoxical sleep is found to occur in association with slow wave sleep when the daily percentage of slow-wave sleep exceeds 15 percent; when the percentage is less than this, no paradoxical sleep occurs (Fig. 5). Lesioning of the rostral nuclei often results in "narcolepsy," with paradoxical sleep following directly upon the waking state. Destructions involving less than 15 percent of the raphe system provoke no significant change in the sleep states. A significant correlation between the volume of the raphe system destroyed and the percentage of sleep was thus demonstrated (47) (Fig. 6).
Biochemical analysis of cat brains in which there had been significant de struction of the raphe system revealed a significant decrease in cerebral serotonin with no variation in the concentration of noradrenalin. Thus, a significant correlation between the volume of the raphe system destroyed, the intensity of the resulting insomnia, and the decrease in cerebral serotonin was demonstrated (Fig. 6) (Table 1). In view of the neuropharmacological findings which showed that inhibition of the synthesis of serotonin (by p-chlorophenylalanine) produces total insomnia and that administration of 5-hydroxytryptophan, the precursor of serotonin, reverses this insomnia, the evidenee favoring the involvement of serotonin in sleep is exeedingly strong.
However, the intrieate and intimate bioehemieal mechanisms underlying the role of serotonin in the sleep process are as yet not well defined. Analysis has failed to reveal a significant change in the concentration of endogenous sero tonin during total deprivation of sleep (48). Nevertheless it has recently been reported that a small but significant fall in serotonin concentration accompanied by a rise in 5-hydroxyindoleacctic acid concentration occurs in the rat hrain during deprivation of paradoxical sleep (49). It has also been demonstrated that the brain concentration of serotonin is higher during the day (when the rat sleeps most of the time) than during the night (22). Only through in vivo study of the tunrnover of serotonin can understanding be achieved of the intricate processes underlying the serotonergic mechanism of sleep.
The Problem of Paradoxical Sleep
The relationship between slow-wave sleep and paradoxical sleep is not a simple one. On the one hand there appears to be a functional link between the two. Thus, after destruction of the raphe system, paradoxical sleep appears only when slow-wave sleep has reached a certain daily threshold ( 15 percent) (Fig. 5). Similarly, following the injection of p-chlorophcnylalanine, the rate of occurrence of paradoxical sleep is closely related to the hrain concentration of serotonin (44). These facts lead to the hypothesis that serotonergic mechanisms involved during slow-wave sleep act as priming mechanisms for the triggering of paradoxical sleep (46). It is probable that these mechanisms are located in the caudal part of thc raphe system, since destruction of the caudal raphe leads to a very severe suppression of paradoxical sleep (relative to slow wave sleep), whereas destruction of th rostral raphe has little effect on paradoxical sleep (which may appear in the absence of slow-wave sleep).
On the other hand it has been revealed that the structures directly responsible for paradoxical sleep are located outside the structures (raphe nuclei) responsible for slow-wave sleep. By suppressing paradoxical sleep through limited lesioning of the dorso ateral pontine tegmentum (a procedure that does not interfere with slow-wave sleep), it was demonstrated that certain pontine structures different from the raphe system are essential to paradoxical sleep (50, 51).
Because monoamine oxidase inhibitors were shown to have the strongest selective suppressing effect upon paradoxical sleep, we undertook the re evaluation of previous results in the light of new findings obtained by histochemical techniques. The nuclei of the locus coeruleus, which were shown to be composed almost exclusively of monoamine oxidase-containing and noradrenalin-containing cells (37,52), were the target (Fig. 4). When a monoamine oxidase inhibitor (nialamide) is administered in the rat or the cat, the resulting selective suppression of paradoxical sleep is found to be correlated with the disappearance of monoamine oxidase staining in the locus coeruleus (29, 53). Moreover, the bilateral destruction of these nuclei produces a total selective suppression of paradoxical sleep (54, 55), whereas control lesions placed rostrally, medially, laterally, or caudally do not affect paradoxical sleep (Table 2). Only lesions immediately ventral to the locus coeruleus nuclei at the level of the nucleus reticularis pontis caudalis which probably involves efferent path ways) suppresses paradoxical sleep (55).
The fact that noradrenalin-containing nerve cells are located in the locus coeruleus suggests that noradrenergic mechanisms may play a role in paradoxical sleep. Destruction of both nuclei decreases the noradrenalin concentration significantly and selectively in rostral parts of the brain (55) (Table 2). In addition, numerous drugs which act upon noradrenalin synthesis may selectively suppress paradoxical sleep (56). This has been shown (57) with alpha-methyl p-tyrosine, which inhibits the synthesis of brain catecholamines at the level of tyrosine hydroxylase (58), and with di sulfiram (59), which impairs the synthesis of noradrenalin at the level of dopamine-beta-hydroxylase (60). On the other hand, alpha-methyl-m-tyrosine and alpha-methyldihydroxyphenylalanine, which may act as false transmitters (61), also have strong suppressive effects on paradoxical sleep in the cat (29, 62). Another indirect evidence of the intervention of a noradrenergic mechanism is the finding that there is an increased turnover of cerebral noradrenalin dur ing the rebound of paradoxical sleep which follows its selective deprivation in the rat (63).
In addition to noradrenalin, cholinergic mechanisms have been implicated in paradoxical sleep by certain pharmacological evidence. Atropine ( 1 to 2 milligrams per kilogram) suppresses paradoxical sleep in the cat (50, 64, 65), whereas eserine may facilitate it in pontile cats (50, 64). Direct injection of acetylcholine or carbachol in the vicinity of the nucleus of the locus coeruleus triggers paradoxical sleep in normal cats (66) . As indicated at the level of the peripheral nervous system (67), it is thus possible that acetylcholine may act as a triggering mechanism for noradrenergic mechanisms.
In sum, neuropharmacological, neurophysiological, and histochemical data provide a partial understanding of the succession of events involved in paradoxical sleep. Priming mechanisms lo ated in the caudal part of the raphe system act upon a target in the dorsolateral pontine tegmentum where densely packed monoamine oxidase containing and noradrenalin-containing neurons are located and where paradoxical sleep is triggered. The occur rence of paradoxical sleep may be inhibited by at least three categories of drugs (Fig. 7). This fact suggests that the paradoxical-sleep mechanism requires three keys in order to operate (deaminated serotonin catabolite, acetylcholine, and noradrenalin). Such a "fail-safe" mechanism contributes to the effectiveness of the organism in that it prevents the intrusion into waking of the hallucinatory processes involved in dreaming.
| Group | Number in Group | Amount of Slow Wave Sleep (%) | Amount of Paradoxical Sleep (%) | % of locus coeruleus destroyed | % of cerebral serotonin | P* | % of cerebral noradrenalin | P* |
| A | 13 | 43+-2 | 9+-0.8 | 0 | 89+-8 | - | 96.5+-8 | - |
| B | 15 | 40+-3 | 0.2+-0.1 | 70+-4.5 | 86+-4 | NS | 43+-6 | .001 |
| C | 4 | 42+-6 | 4.3+-1 | 27+-5 | 120+-4 | NS | 85+-11 | NS |
| D | 6 | 46+-4 | 8.5+-2 | 11+-6 | 73+-9 | NS | 78+-4 | NS |
* Student's t-test values for P obtained by comparison with group A; NS; not significant. Each P column refers to the column that immediately precedes it.
Table 2. comparison, for various groups of cats with brain lesions, of the amounts of slow wave sleep and paradoxical sleep, expressed as (i) a percentage of recording time (10 to 13 days); (ii) the percentage of the nucleus locus coeruleus destroyed surgically; and (iii) the amounts of serotonin and noradrenalin in the brain rostral to the lesion, expressed as percent ages of the amounts in the brains of normal control cats. The group divisions are as follows: (A) cats that had undergone sham operations, used as controls in Student's t-test, (s) cats with total or subtotal hilateral lesions of the nucleus locus coeruleus that resulted in total suppression of paradoxical sleep; (c) cats with lesions lateral to the nucleus locus coeruleus involving the nuclei parahrachialis medialis and lateralis; (D) cats with lesions caudal to the nucleus locus coeruleus involving the vestibular nuclei. The percentages are mean values for an entire group, plus standard deviation.
Summary
Recent advances in the physiology of sleep have led to the understanding that sleep is comprised of two succes sive functional states (slow-wave sleep and paradoxical sleep) which depend upon active mechanisms. These two states can be accurately quantified, and selectively modified or suppressed by either specific drugs or limited les ions.
The hypothesis that cerebral serotonin has a role in the process of sleep is strongly supported by two series of experiments. (i) Inhibition of the synthesis of serotonin at the level of tryptophan hydroxylase by p-chlorophenylalanine leads to total insomnia which is reversible; return to normal sleep is effected by the injection of 5-hydroxytryptophan, the immediate precursor of serotonin. (ii) Total destruction of serotonin-containing neurons located in the raphe system (as determined by histofluorescence ) also leads to total insomnia. A three-way correlation exists between the extent of destruction of the raphe, the decrease in cerebral serotonin, and the resulting insomnia.
Paradoxical sleep appears to depend upon "priming" serotonergic mecha nisms located in the caudal raphe system and upon "triggering" mechanisms located in the nuclei of the locus coeruleus. Destruction of these nuclei leads to the suppression of paradoxical sleep without alteration of slow-wave sleep. The successive intervention of serotonergic, cholinergic, and noradrenergic mechanisms in the triggering and effecting of paradoxical sleep is strongly implied by neuropharmacological results.
Figure 1 : Polygraphic recording of the adult cat, showing waking and the two states of sleep
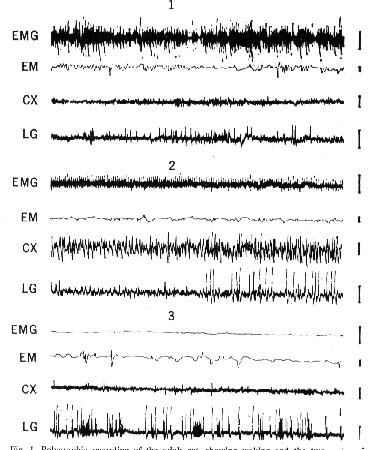
- Waking: muscular activity of the neck (EMG), eye movements (EM), fast low-voltage activity of the occipItal. cortex (CX), and sharp waves in the lateral genicu late (LG) which accompany eye movements.
- Slow-wave sleep: decreased muscular activity of the neck, absence of eye movements, high-voltage slow waves on the cortex, and high-voltage geniculate waves which directly precede paradoxical sleep by 1 or 2 minutes
- Paradoxical sleep: total disappearance of EMG activity. together with rapid eye movements, fast low-voltage cortical activity. and clusters of high-voltage lateral geniculate waves. Each line represents l minute of recording; amplitude calibra tion, 50 microvolts.
Figure 2 : Effects of p-chlorophenylalanine on sleep and brain concentrations of serotonin in the rat
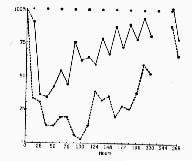
After intraperitoneal injection (500 milligrams per kilogram), there is a decrease in the amount of slow wave sleep (SWS) and in serotonin (5 HT) concentration, followed by a slow return to normal values after 268 hours.
(Solid line) Percentage of slow-wave sleep relative to the amount for a control rat; each point represents the mean percentage per 12-hour period (black dots at top in dicate night hours, 7 p.m. to 7 a.m. ) .
(Dashed line) Percentage of serotonin (relative to the concentration for a control rat); for the purpose of this analysis, two animals were killed every 12 hours.
Figure 3 : Effect of secondary injection of 5-hydroxytryptophan during insomnia produced by p-chlorophenylalanine in the cat
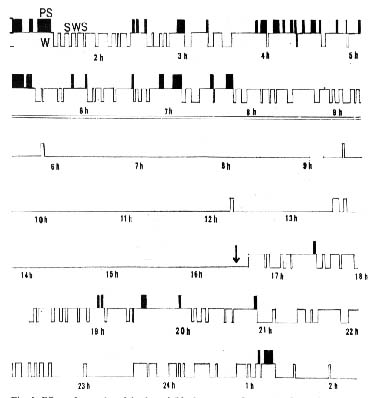
(Top two lines) Normal pattern of sleep in a cat for which electroencephalographic recordings were made over a long period. (Bottom five lines) Total insomnia 96 hours after the last of three daily injections of p-chloro phenylalanine (400 milligrams per kilogram), then, following the injection (see arrow) of 5-hydroxytryptophan (5 milligrams per kilogram), reappearance of the normal pat tern of sleep. Insomnia returns 8 hours after the injection. (Base line of each record) Waking; (white rectangles) slow-wave sleep: (black rectangles) paradoxical sleep.
Figure 4 : Subtotal lesion of the raphe system in the cat
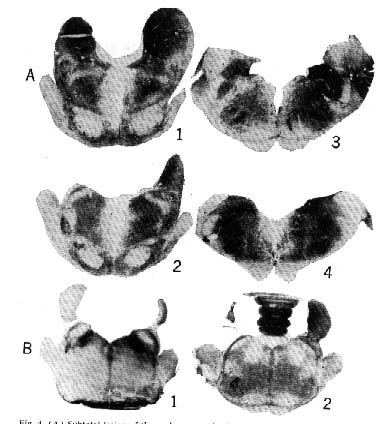
Rostral section at the level of ( 1) the raphe centralis superior and dorsalis nuclei, (2) the raphe pontis, and (3 and 4) the raphe magmls and pallidus. The mean percentage of slow-wave sleep for this particular cat was 8 percent; it manifested no paradoxical sleep during 12 days of recording. (B) Rostral sections of the pons: ( 1) Glenner coloration shows the monoamine oxidase (dark areas) in the nucleus locus coeruleus, in the nuclei parabrachialis medialis and lateralis, and, to a lesser extent, in the raphe system (2) bilateral destruction of the nuclells locus coeruleus which selectively suppresses paradoxical sleep.
Figure 5 : Relationships between paradoxical sleep and slow wave sleep
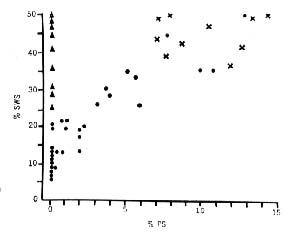
(Crosses) Cats that have undergone sham operations; the values for slow-wave sleep and paradoxical sleep are normal. (Black dots) Cats with the raphe system totally or partially destroyed. Note that paradoxical sleep occurs only if the amount of slow-wave sleep is greater than 15 percent. (Triangles) Cats with total bilateral destruction of the nucleus locus coeruleus. Values for slow-wave sleep are normal; paradoxical sleep is com pletely suppressed.
Figure 6 : Three-way correlation of the extent of the destruction of the raphe system
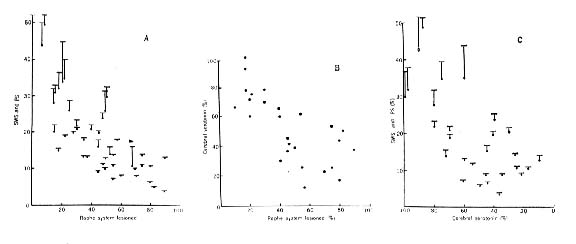
(A and B), the level of serotonin in the brain rostral to the lesion (B and C), and the resulting diminution of both states of sleep (A and C). In A and C, total sleep is represented by dots; paradoxical sleep, by a vertical line; total sleep, by a horizontal bar.