Pierre-Hervé Luppi*, Christelle Peyron, Claire Rampon, Damien Gervasoni, Bruno Barbagli, Romuald Boissard and Patrice Fort
Address : Laboratoire de Médecine Expérimentale, CNRS ERS 5645 and INSERM U52, Faculté de Médecine, 8 avenue Rockefeller, 69373 LYON cedex 08, FRANCE
4.Conclusions and new hypothesis*
In the mammalian central nervous system, main groups of {the "majority" means more than one half} noradrenergic and serotonergic neurons are found within the locus coeruleus (LC) and the dorsal raphe nucleus (DRN), respectively.1 By means of their widespread projections throughout the entire brain, these monoaminergic neurons are thought to play crucial roles in a great variety of physiological and behavioral functions including sleep and wakefulness.2-9 Accordingly, extracellular electrophysiological recordings in freely moving rats and cats have shown that LC noradrenergic and DRN serotonergic neurons fire tonically during wakefulness (W), decrease their activity during slow wave sleep (SWS), and are nearly quiescent during paradoxical sleep (PS) (PS-off cells).5-9 It has been proposed that the spontaneous tonic firing of LC and DRN neurons during W, which is close to that observed in anesthetized rats,10 is mainly due to their intrinsic pacemaker properties, as revealed by intracellular recordings from LC neurons in slices10,11 and cultures.12
The mechanisms responsible for the decrease of activity of the monoaminergic neurons during SWS are not well known. However, the classical view is that the GABA-ergic transmission is enhanced during SWS as supported by the strong hypnotic properties of the benzodiazepines acting on the GABAA receptors (reviewed in13-14). Moreover, Nitz and Siegel recently found an increase in the amount of GABA in the cat LC during SWS compared to W.15-16 Although similar increases in GABA were not found in the DRN, Levine and Jacobs showed that iontophoretic application of bicuculline, a GABAA antagonist, on DRN serotonergic neurons reversed the typical suppression of activity seen during SWS.17 GABA-induced inhibition might therefore likely be responsible for the decrease of activity of monoaminergic neurons during SWS.
The cessation of firing of these neurons during PS, according to the classical "reciprocal interactions" models, is the result of active PS-specific inhibitory processes originating from the pontine neurons responsible for PS onset and maintenance (PS-on cells).7,18 These neurons were first thought to be cholinergic and localized in the dorsal pons (laterodorsal tegmental, pedunculopontine and peri-LCa nuclei). It has later been suggested that they might use GABA or glycine (GLY), rather than acetylcholine, as an inhibitory neurotransmitter.19,20 Indeed, acetylcholine excites LC noradrenergic neurons and is only weakly inhibitory on serotonergic DRN neurons.21,22 In contrast, in anesthetized rats, iontophoretic application of GABA or GLY strongly inhibits LC and DRN neurons, and co-iontophoresis of bicuculline or strychnine (GABAA and GLY antagonists, respectively) antagonizes these effects.19,23-25 Furthermore, in vitro studies on slices using focal stimulation and bath-application of bicuculline and strychnine revealed GABA- and GLY-mediated IPSPs in LC neurons, and GABA-mediated IPSPs in DRN cells.26-29 In agreement with these results, GABA-and GLY-immunoreactive varicose fibers as well as GABAA and GLY receptors have been found in the rat LC and DRN19,20,30-32. Supporting the hypothesis that glycinergic neurons are responsible for the inhibition of monoaminergic neurons during PS, it has been shown that glycine was responsible for the inhibition of the somatic motoneurons during pS 33. In contrast, supporting a role for GABA, microinjections of picrotoxin (a GABAA antagonist) in rat LC significantly reduced the duration of PS episodes.34
In conclusion, several lines of evidence suggested to us that GABA and/or GLY might be responsible for the inhibition of monoaminergic neurons during SWS and PS. To test this hypothesis: 1) we applied bicuculline or strychnine on LC noradrenergic cells during SWS, PS and W using a new method which allows extracellular single-unit recordings of neurons combined with iontophoresis in the head-restrained unanesthetized rat,35 and 2) we localized in rats the glycinergic and GABA-ergic neurons potentially responsible for the inhibition of the monoaminergic neurons of the DRN and LC, by combining injections of the retrograde tracer cholera-toxin B subunit (CTb) in the DRN and LC and immunohistochemistry for GLY or glutamic acid decarboxylase (GAD; the synthetic enzyme for GABA)
2 - Effects of the application of GABA and glycine antagonists on the activity of locus coeruleus neurons during sleep
2.1 - Spontaneous activity of LC neurons during the sleep-waking cycle
LC neurons were recorded during at least two of the three basic vigilance stages. Noradrenergic LC neurons were identified by their typical broad action potentials (1.5-2 ms duration), and a phasic excitatory response to a sensory stimulus (audible click) immediately followed by an inhibition36-38. Their mean discharge rate was 1.39 Hz during quiet W. During SWS, LC cells showed a decrease in their firing rate (0.56 Hz) while during PS episodes they were nearly quiescent (0.01 Hz) showing only occasional single spikes 39.
2.2 - Iontophoretic applications of strychnine
Iontophoretic ejections of strychnine (50-250 nA, 20-100 sec) induced a sustained increase in the discharge rate of LC neurons regardless of the vigilance state: from 1.70 to 11.41 during W, 0.39 to 9.34 Hz during SWS (Fig. 1A), and 0.06 to 14.13 Hz during PS (Fig. 1B).35 Occasionally, the animal displayed successive short periods of SWS and W after strychnine administration while the firing rate of the recorded neuron was still elevated. In these instances, we compared the neuron’s increase in discharge rate during two successive SWS and W periods. We found that the increase in neuronal activity was 1.93 times as great during W as during SWS periods. This difference was statistically significant.
In all neurons tested, the strychnine effects appeared 9-93 sec after the onset of iontophoretic application. Recovery to baseline activity occurred several tens of seconds (range: 20-280 sec) after the termination of strychnine ejection. Like in anesthetized animals, 19,23 iontophoretic applications of glycine or GABA suppressed the spontaneous discharge of LC neurons during W or SWS, and glycine- but not GABA-induced inhibitions were antagonized by co-iontophoresis of strychnine.
2.3 - Iontophoretic applications of bicuculline
Iontophoretic ejections of bicuculline during W, SWS and PS (30-200 nA, 20-570 sec) induced a progressive and sustained increase in the discharge rate of LC neurons without inducing a change in vigilance state.39 The average firing frequency increased from 1.09 to 8.72 Hz during W (Fig. 2B) and from 0.34 to 6.91 Hz during SWS (Fig. 2A). During PS, LC neurons were practically silent (0.01 Hz); however, following bicuculline application they showed a remarkably high mean firing rate of 6.59 Hz (Fig. 2B). In recording some neurons, the rat displayed successive short periods of SWS and W while the effect of bicuculline on neurons was still present. With these neurons, we compared the increase in discharge rate during two successive SWS and W periods. We found that the increase in activity was 1.23 times as great in W as in SWS periods. In contrast with strychnine, this difference was not statistically significant. Discharge rate increases occurred 20-70 sec after the beginning of iontophoretic applications, while the recovery to baseline activity occurred several tens of seconds (range: 20-400 s) after the end of ejection.
During W or SWS, GABA-induced inhibitions were fully antagonized by bicuculline co-iontophoresis, while GLY-induced inhibitions were unaffected (Fig. 2B).
2.4 - Conclusions: Pharmacological and physiological significance
Iontophoretic applications during PS or SWS of bicuculline or strychnine induced a tonic firing in LC noradrenergic neurons. In addition, applications of bicuculline or strychnine during wakefulness induced a sustained increase in discharge rate. These results seem to indicate the existence of tonic GABA and glycinergic inputs to the LC that are active during all vigilance states. However, it is conceivable that these effects are due to non-specific excitatory effects of strychnine and bicuculline. Several arguments are against this possibility, however. With respect to bicuculline, only a slight increase in LC neuron’s firing rate has been observed under anesthesia following iontophoretic or pressure applications of bicuculline.23,40 In addition, bicuculline applications with parameters similar to those used in our studies in anesthetized rats or cats induced an increase in discharge rate of neurons from the medial hypothalamus,41 lateral geniculate nucleus,42 dorsal periaqueductal gray,43 nucleus of the solitary tract,44-46 cochlear nuclei47 and the spinal cord.48,49 Finally, intracellular recordings of hippocampal or cortical neurons in slices revealed the presence of spontaneous GABA-mediated IPSPs.50,51 Taken together, these results suggest that, like many different types of CNS neurons, the LC noradrenergic cells are tonically inhibited by GABA.
It is also unlikely that the strong and long-lasting excitations observed after strychnine applications are due to nonspecific and/or toxic effects. Indeed, if this was the case, we should have also seen a strong excitation following strychnine applications in LC neurons in anesthetized animals. In contrast, under halothane or choral hydrate anesthesia, we saw only a slight increase in LC neuron’s firing rate even with ejection currents up to 350 nA.19. Moreover, it has been shown that strychnine applications with similar parameters have no direct excitatory effects on other types of neurons. Thus, Bennett et al.,44 Sun and Guyenet52 and Jordan et al.46 have shown that iontophoretic applications of strychnine do not affect the spontaneous or evoked activity of cardiovascular related neurons in the nucleus of the solitary tract or the lateral paragigantocellular nucleus. It has also been shown that applications of strychnine on inspiratory neurons from the nucleus of the solitary tract induce an activation specifically at the end of expiration.45 Finally, following strychnine iontophoretic ejections on intracellularly recorded spinal motoneurons, no direct excitatory or toxic effects were reported.33
In conclusion, the effects of strychnine and bicuculline seem to indicate the existence of tonic glycinergic and GABA-ergic inputs to LC cells during W, SWS and PS. Further, we found that when the same neuron was recorded during short successive periods of SWS and W while the strychnine effect on the neuron was still present, its increase in discharge rate was much greater during W than during SWS. In contrast, in the same situation but after bicuculline administration, the increase of discharge rate of a given neuron was not statistically different between W and SWS. These results strongly suggest that release of GABA but not GLY is responsible for the inactivation of LC noradrenergic neurons during SWS. Unfortunately, due to the smaller number of LC cells recorded during PS, we were not able to make the same comparison between SWS and PS. However, with the microdialysis technique, Nitz and Siegel recently found an increase in GABA release in the cat LC during SWS as compared to waking values, and a further increase during PS, and, in contrast, no detectable changes in GLY concentrations.16 Based on these and our results, we therefore suggest that during W, the LC cells are under a tonic GABA-ergic inhibition which increases during SWS and even further during PS, and that the increase in GABA-ergic inhibition is at least partly responsible for the inactivation of these neurons during the sleep states. In contrast, the glycinergic tonic inhibition would be constant across the sleep-waking cycle and, thus, control the general excitability of LC neurons.
It remains to be determined whether the tonic GABA-ergic and glycinergic inhibitions revealed in this study involve post- and/or presynaptic mechanisms Indeed, the activation of LC cells seen after bicuculline or strychnine might be due not only to a removal of postsynaptic effects of GABA or GLY inputs but also to that of GABA-ergic or glycinergic presynaptic inhibition of excitatory inputs to LC cells. Such presynaptic GABAergic inhibition of excitatory terminals has been well documented for many neurons in the CNS53 and is therefore likely to occur in LC noradrenergic neurons as well. In contrast, glycinergic presynaptic inhibition remains to be demonstrated in CNS neurons.
Concerning the DRN serotonergic neurons, the available data are somewhat contradictory. Indeed, Nitz and Siegel reported a significant increase in GABA release in the DRN during PS as compared to W and SWS, with no difference between W and SWS.15 In contrast, Levine and Jacobs found that the iontophoretic application of bicuculline reversed the typical suppression of neuronal activity during SWS but not during PS.17 Additional experiments are therefore necessary to determine whether GABA or GLY plays a role in the inactivation of DRN serotonergic cells during SWS and PS.
3 - Glycinergic and GABA-ergic afferent projections to the Locus Coeruleus and Dorsal Raphe nucleus
3.1 - Double immunostaining procedures
The procedures used for retrograde tracing with cholera-toxin B subunit (CTb) and anterograde tracing with phaseolus vulgaris leucoagglutinin (PHAL) have been described in detail previously.54-56 The high sensitivity of CTb allowed us to demonstrate pathways not seen before using other tracers including horseradish peroxidase (HRP), wheatgerm agglutinin conjugated HRP (WGA-HRP) and fluorescent tracers.
3.2 - Afferent projections to the LC
Following iontophoretic injections of CTb into the core of the LC (Fig. 3A), we observed a substantial number of retrogradely labeled cells in the lateral and dorsal paragigantocellular nuclei as previously described.5
We also saw a substantial number of retrogradely labeled neurons in (1) the preoptic area dorsal to the supraoptic nucleus, (2) areas of the posterior hypothalamus, (3) the Kölliker-Fuse nucleus, (4) and the mesencephalic reticular formation. Fewer labeled cells were also observed in other regions including the hypothalamic paraventricular nucleus, the dorsal and median raphe nuclei, dorsal part of the periaqueductal gray, the area of the noradrenergic A5 group, the lateral parabrachial nucleus and the caudoventrolateral reticular nucleus.
These results were confirmed and further extended by the anterograde transport data with CTb and PHAL. Injections of these tracers in the lateral paragigantocellular nucleus, preoptic area dorsal to the supraoptic nucleus, the posterior hypothalamic areas, the ventrolateral part of the periaqueductal gray, and the Kölliker-Fuse nucleus yielded a substantial to large number of labeled fibers in the nuclear core of the LC.
In conclusion, our results indicate that the LC receives afferents from a very large number of structures from the forebrain to the medulla. Failure to demonstrate these afferents in earlier works 5,57 was most likely due to the poor sensibility of the retrograde tracers used (HRP, WGA-HRP and fluorogold).
3.2.1 - Origin of the glycinergic input to the LC
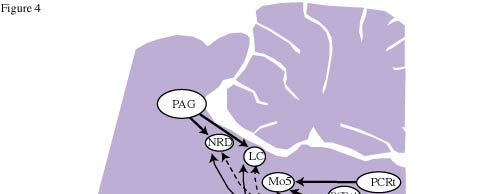
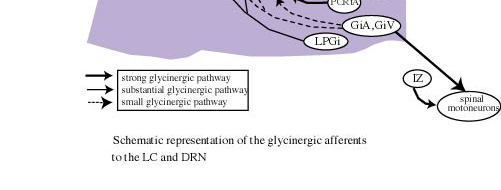
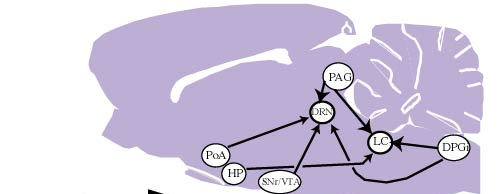
Following CTb injections centered at the LC, a large number of cells that were positive for both CTb and GLY immunoreactivity were observed in the ventrolateral and lateral parts of the periaqueductal gray, where they represented a small proportion of the CTb-positive cells (Fig. 3D). Also at the same level, a substantial number of double-labeled neurons were detected in the mesencephalic reticular formation. A moderate number of double-labeled neurons were seen in the nucleus raphe magnus, gigantocellular alpha, and lateral and dorsal paragigantocellular nuclei.58 These results are summarized in Figure 4.
3.2.2 - Origin of the GABA-ergic input to the LC
Following CTb injections in the LC, many brain regions contained neurons that are positive for both CTb and GAD immunoreactivity. A large number of double-labeled cells were localized in the lateral preoptic area, the lateral hypothalamic area, the lateral and ventrolateral parts of the periaqueductal gray and the dorsal paragigantocellular nucleus. A small number of double-labeled cells were also seen in the dorsal hypothalamic area, the tuberomammillary nucleus, the mesencephalic reticular formation, the lateral parabrachial nucleus and the nucleus raphe magnus.59 These results are summarized in Figure 4.
3.3 - Afferent projections to the DRN
We confirmed that the lateral habenula contains a large number of retrogradely labeled cells following CTb injections in the DRN (Fig. 3B).60 In addition, we observed a large number of retrogradely labeled cells in the orbital, cingulate, infralimbic, dorsal peduncular, and insular cortices. A substantial number of retrogradely labeled cells were also visible in the ventral pallidum, claustrum, bed nucleus of the stria terminalis, medial and lateral preoptic areas, medial preoptic nucleus, lateral, dorsal and posterior hypothalamic areas, zona incerta, subincertal nucleus, tuber cinerium cinereum and medial tuberal nucleus.
In the brainstem, a large number of neurons were found in the ventral part of the periaqueducal gray, just above the oculomotor nuclei, and more caudally in the ventrolateral, lateral and dorsal parts of the periaqueductal gray. At the same level, the mesencephalic and oral pontine reticular nuclei contained numerous CTb-positive cells. At pontine levels, the largest number of CTb-positive cells were seen in the lateral parabrachial nucleus. The medial parabrachial nucleus, the lateral tegmental nucleus of Castaldi and the pontine periaqueductal gray contained a moderate number of CTb-positive cells. At medullary levels, only a small number of neurons were found in the nucleus raphe magnus, as well as parvocellular, gigantocellular alpha, lateral and dorsal paragigantocellular nuclei, and the nucleus of the solitary tract.
3.3.1 - Origin of the glycinergic input to the DRN
Following CTb injections in the DRN, many medium-sized cells that were positive for CTb and GLY were found bilaterally in the ventrolateral and lateral parts of the periaqueductal gray caudal to the trochlear nucleus, and in the adjacent mesencephalic reticular formation. A few small, double-labeled cells were also found in a rostral periaqueductal region dorsal to the oculomotor complex. The double-labeled cells in the periaqueductal gray represented a small proportion of CTb-labeled neurons in that region.
In the medulla, a moderate number of double-labeled cells were seen within the lateral paragigantocellular nucleus, the nucleus raphe magnus and the gigantocellular reticular alpha nucleus.58 These results are summarized in Figure 4.
3.3.2 - Origin of the GABA-ergic input to the DRN
Neurons immunoreactive for GAD and serotonin in the DRN
After double immunostaining of GAD (in brown) and serotonin (in black) on the same sections, GAD-immunoreactive cell bodies were observed in the lateral part of the DRN mixed with serotonergic neurons. In contrast, the medial part of the DRN contained a large number of serotonergic cells and only a few GAD-positive neurons. Double-labeled cells were not observed. These results fit perfectly with the recent findings of Stamp and Semba.61
GAD and CTb-immunoreactive neurons
Following CTb injections in the DRN, the largest number of cells that were positive for both CTb and GAD were localized in the lateral hypothalamic area. A substantial number of double-labeled cells were also observed in the medial and lateral preoptic areas, the substantia nigra pars reticulata, the ventral tegmental area, and the ventrolateral periaqueductal gray. A moderate number of double-labeled cells were seen in the ventral pallidum, the parabrachial area, the oral pontine reticular nucleus and the dorsal paragigantocellular nucleus. Finally, a small number of double-labeled neurons were seen in the paraventricular hypothalamic area, the lateral habenula, and the tuberomammillary, raphe magnus and gigantocellular alpha nuclei59 .These results are summarized in Figure 4.
3.4 - Physiological role of the glycinergic inputs to the DRN and the LC
We found a major glycinergic input to the LC and the DRN from the ventrolateral and lateral parts of the periaqueductal gray and the adjacent mesencephalic reticular formation. The lateral paragigantocellular, raphe magnus and gigantocellular alpha nuclei provide additional small glycinergic inputs to the LC and the DRN. The LC receives a small additional projection from the dorsal paragigantocellular nucleus. These results first indicate that the glycinergic inputs to the DRN and to the LC arise from the same structures and therefore might share the same functions for monoaminergic neurons contained in these two nuclei. Of further interest regarding these results, we found a tonic glycinergic inhibition of LC neurons during the entire sleep waking cycle. In addition, no change in glycine levels in the DRN and LC has been reported across the sleep-waking cycle by Nitz and Siegel.15,16 It can therefore be proposed that glycine release onto LC and DRN cells might be constant during all vigilance states and serve to dampen their excitability generally, rather than exerting selective inhibition during sleep. This control should mainly arise from the periaqueductal gray, which is the main source of GLY to the DRN and LC. This proposal does not apparently fit with the recent results of Sastre et al. 62 in cats showing that the inactivation of the ventrolateral periaqueductal gray by muscimol (a GABAa agonist) induced a dramatic increase of PS. Such contradiction might be explained by the fact that the glycinergic neurons constitute only a minor proportion of the neurons in the ventrolateral periaqueductal gray. The effect seen by Sastre et al. could therefore be due to the inhibition of other types of neurons, in particular glutamatergic or GABAergic neurons63.
In addition to glycinergic neurons in the periaqueductal gray, we observed a small number of glycinergic neurons in the nucleus gigantocellular alpha and the adjacent raphe magnus projecting to the DRN and LC. Based on a number of studies in cats, we previously proposed the hypothesis that during PS, the monoaminergic neurons and the cranial and spinal somatic motoneurons might be inhibited by a single population of glycinergic neurons located in the magnocellular reticular nucleus (cat’s equivalent of gigantocellular alpha and ventral nuclei). However, the present results and those we recently obtained on the glycinergic afferents to the motor trigeminal nucleus64 indicate that at least in rats the gigantocellular alpha and ventral nuclei provide only a limited glycinergic input to the DRN, LC and motor trigeminal nucleus, and therefore are unlikely to contain a single population of neurons responsible for the inhibition of monoaminergic and all somatic motoneurons during PS. In fact, in rats, the only motoneurons receiving a strong glycinergic projection from the gigantocellular alpha and ventral nuclei are those of the spinal cord as demonstrated by Holstege and Bongers.65 In view of these results in rats, the cranial motoneurons might be inhibited during PS by neurons from the parvocellular and parvocellular alpha reticular nuclei, and glycine might not selectively be involved in the inhibition of monoaminergic neurons during sleep.64 Additional experiments are nevertheless necessary to confirm such conclusion in particular in cats in which it has been shown that the magnocellular reticular nucleus but not the parvocellular reticular nuclei contains c-Fos positive cells following long periods of PS induced by carbachol injections in the pons.66
3.5 - Physiological role of the GABA-ergic inputs to the DRN and the LC
Our results indicate that the LC and DRN receive GABA-ergic inputs from neurons located in a large number of distant regions from the forebrain to the medulla (Fig. 4). We also observed a substantial number of GAD-immunoreactive neurons in the pontine and mesencephalic periaqueductal gray that project to the LC and DRC. These results indicate that the GABA innervation of these two monoaminergic nuclei arises from multiple, distant GABA-ergic groups in addition to local GABAergic neurons. Such results contrast with the classical concept that GABA is mainly contain in interneurons. They suggest that the serotonergic neurons of the DRN and noradrenergic neurons of the LC could be inhibited by multiple populations of GABA-ergic neurons located in different structures, and raises the question of the functional significance of such complexity. One possibility is that only some of these GABA-ergic afferents are destined to the serotonergic neurons of the DRN and the noradrenergic neurons of the LC. This seems likely for the DRN which is an heterogeneous structure, but not for the LC which in rats contains nearly exclusively noradrenergic cells. Another possibility is that some of these afferents are postsynaptic and the others presynaptic, but the more likely explanation is that each of these afferents is active only under specific physiological conditions.
Based on physiological and electrophysiological data (see above), we expect that one or several of these GABA-ergic afferents are "turned on" specifically at the onset and during SWS, while one or several others are "turned on" specifically at the onset and during PS. These afferents would thus be respectively responsible for the progressive decrease of activity of monoaminergic neurons from W to SWS and from SWS to PS. Among the GABA-ergic structures revealed in our study, several are common to the DRN and the LC and are therefore good candidates for these roles.
The most likely candidate for the inhibition of the monoaminergic neurons during SWS is the lateral preoptic area. Indeed, lesion of this structure in cats and rats induced an insomnia while its stimulation induced SWS.67-72 Neurons increasing their activity during SWS have been recorded in this area.73-75 Moreover, c-Fos positive cells were observed in the lateral preoptic area after long periods of SWS 76 and it has been further shown that these neurons are in part GABA- and galanin-positive and project to the tuberomammillary nucleus which contains waking-active, presumably histaminergic neurons.77 From these and our results, we can therefore propose that GABA-ergic neurons in the lateral preoptic area increase their firing just before the onset and during SWS and induce SWS via their inhibitory projections to waking-inducing structures (tuberomammillary nucleus, DRN and LC among others).
In addition to the lateral preoptic area, the lateral hypothalamic area, the periaqueductal gray and the dorsal paragigantocellular nucleus provide substantial GABA inputs to the DRN and LC. The strong GABA-ergic projection from the lateral hypothalamic area is rather puzzling. Indeed, since the initial demonstration that the lesion of the posterior hypothalamus induces somnolence,78 the histaminergic neurons of the posterior hypothalamus have been more specifically implicated in waking.79 These neurons are located in the tuberomamillary nucleus, ventral and caudal to the GABA-ergic neurons in the lateral hypothalamic area that project to the DRN and LC. Nevertheless, muscimol injections in cats in the lateral hypothalamic area in addition to those in the tuberomammillary nucleus induced hypersomnia.80 Additional experiments are therefore needed to determine whether the lateral hypothalamic area and its GABA-ergic neurons play a role in vigilance control via their projections to the DRN and LC.
The GABAergic afferents responsible for the inhibition of monoaminergic neurons during PS should be located in the brainstem. Indeed, it is well known that PS-like episodes occur in pontine or decerebrate cats.2 Moreover, it has recently been shown that, in decerebrate animals, PS episodes induced by carbachol injections in the pons are still associated with a cessation of activity of serotonergic neurons of the raphe obscurus and pallidus nuclei.81.
In the brainstem, we observed substantial GABA-ergic projections to the LC and DRN from the periaqueductal gray and the dorsal paragigantocellular nucleus. In agreement with these results, local application of bicuculline blocked the dorsal paragigantocellular-evoked inhibition of LC neurons.23 In addition, recent findings on slices showed that focal iontophoretic application of NMDA in the ventral periaqueductal gray induced bicuculline sensitive IPSPs in DRN serotonergic neurons.82 The GABAergic afferents from the periaqueductal gray and the dorsal paragigantocellular nucleus could therefore be responsible for the inhibition of monoaminergic neurons during PS.
The hypothesis that this inhibition is coming from neurones located in the periaqueductal gray is further supported by two recent studies. Yamuy et al. showed that after a long period of PS induced by pontine injection of carbachol, a large number of c-Fos positive cells are visible in the DRN and a region lateral to it.83. Moreover, Maloney and Jones84 observed, after a PS rebound induced by deprivation, an increase in c-Fos-positive GAD immunoreactive neurons in the periaqueductal gray and the lateral tegmental nucleus.
Finally, it must be acknowledged that although a number of arguments are in favor of a role of GABA in the inhibition of monoaminergic neurons during SWS and PS, other inhibitory neuroactive substances might also participate in this inhibition. Indeed, it has for example been shown that LC and DRN cells are inhibited by local application of enkephalin.82,84 Moreover, it has been shown that extracellular adenosine levels increase during wakefulness and decrease during SWS,86 and that adenosine application is inhibitory on LC noradrenergic cells.87,88
The removal or reduction during SWS or PS of tonic excitatory inputs that may be present at high levels during W (e.g., acetylcholine or glutamate) might also contribute to the decrease of activity of LC and DRN neurons during sleep.
4 - Conclusions and new hypothesis
During SWS, the monoaminergic neurons would be inhibited by GABA-ergic neurons located in the lateral preoptic area. At the onset of and during PS, a second population of GABA-ergic neurons located in the periaqueductal gray or the dorsal paragigantocellular nucleus would be responsible for the complete cessation of the monoaminergic neurons during this sleep state. The cranial motoneurons would be inhibited by glycinergic neurons of the parvocellular nucleus and those of the spinal cord by glycinergic neurons from the gigantocellular ventral nucleus and glycinergic interneurons of the intermediate zone of the spinal cord.
ACKNOWLEDGMENTSThis work was supported by INSERM (U52), CNRS (ERS 5645), Université Claude Bernard Lyon I and the 1996 ESRS-Synthélabo European Research Grant. The authors wish to thank C. Guillemort (GFG Co, Pierre-Bénite) for his help in designing the head-restraining system.
References
- 1 Dahlström, A. and Fuxe, K., Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration in the cell bodies of brain stem neurons, Acta Physiol. Scand. (Suppl.), 232, 1, 1964.
- 2 Jouvet, M., The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep, Ergebn. Physiol., 64, 166, 1972.
- 3 Jacobs, B.L., Wilkinson, L.O. and Fornal, C.A., The role of brain serotonin. A neurophysiologic perspective, Neuropsychopharmacol., 3, 473, 1990.
- 4 Jacobs, B.L. and Azmitia, E.C., Structure and function of the brain serotonin system, Physiol. Rev., 72, 165, 1992.
- 5 Aston-Jones, G., Ennis, M., Pieribone, V.A., Nickell, W.T. and Shipley, M.T., The brain nucleus locus coeruleus : restricted afferent control of a broad efferent network, Science, 234, 734, 1986.
- 6 Aston-Jones, G. and Bloom, F.E., Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle, J. Neurosci., 1, 876, 1981.
- 7 Hobson, J., McCarley, R. and Wyzinski, P., Sleep cycle oscillation : reciprocal discharge by two brainstem groups, Science, 189, 55, 1975.
- 8 McGinty, D.J. and Harper, R.M., Dorsal raphe neurons : depression of firing during sleep in cats, Brain Res., 101, 569, 1976.
- 9 Trulson, M.E. and Jacobs, B.L., Raphe unit activity in freely moving cats : correlation with level of behavioral arousal, Brain Res., 163, 135, 1979.
- 10 Aghajanian, G.K. and VanderMaelen, C.P., Intracellular identification of central noradrenergic and serotonergic neurons by a new double labeling procedure, J. Neurosci., 2, 1786, 1982.
- 11 Williams, J.T., North, R.A., Shefner, A., Nishi, S. and Egan, T.M., Membrane properties of rat locus coeruleus neurons, Neuroscience, 13, 137, 1984.
- 12 Masuko, S., Nakajima, Y., Nakajima, S. and Yamaguchi, K., Noradrenergic neurons from the locus ceruleus in dissociated cell culture : culture methods, morphology, and electrophysiology, J. Neurosci., 6, 3229, 1986.
- 13 Mendelson, W.B., Neuropharmacology of sleep induction by benzodiazepines, Crit. Rev. Neurobiol., 622, 1, 1992.
- 14 Gaillard, J.-M., Principles and Practice of Sleep Medicine, M.H. Kryger, T. Roth and W.C. Dement (eds), Philadelphia, 1994, 349.
- 15 Nitz, D.A. and Siegel, J.M., Inhibitory amino acid neurotransmission in the dorsal raphe nucleus during sleep/wake states, Abstr. Soc Neurosci., 19, 1815, 1993.
- 16 Nitz, D.A. and Siegel, J.M., GABA release in the locus coeruleus as a function of sleep/wake state, Neuroscience, 78, 795, 1997.
- 17 Levine, E.S. and Jacobs, B.L., Neurochemical afferents controlling the activity of serotonergic neurons in the dorsal raphe nucleus : microiontophoretic studies in the awake cat, J. Neurosci., 12, 4037, 1992.
- 18 Sakai, K., Sleep : Neurotransmitters and Neuromodulators, A. Wauquier, J.M. Monti, J.M. Gaillard and M. Radulovacki (eds), New York, 29, 1985.
- 19 Luppi, P-H, Charlety, P.J., Fort, P., Akaoka, H., Chouvet, G. and Jouvet, M., Anatomical and electrophysiological evidence for a glycinergic inhibitory innervation of the rat locus coeruleus, Neurosci. Lett., 128, 33, 1991.
- 20 Jones, B.E., Noradrenergic locus coeruleus neurons : their distant connections and their relationship to neighboring, including cholinergic and GABA-ergic neurons of the central gray and reticular formation, Prog. Brain Res., 88, 15, 1991.
- 21 Guyenet, P.G. and Aghajanian, G.K., Ach, Substance P and Met-Enkephalin in the locus coeruleus : pharmacological evidence for independent sites of action, Eur. J. Pharmacol., 53, 319, 1979.
- 22 Koyama, Y. and Kayama, Y., Mutual interactions among cholinergic, noradrenergic and serotonergic neurons studied by iontophoresis of these transmitters in rat brainstem nuclei, Neuroscience, 55, 117, 1993.
- 23 Ennis, M. and Aston-Jones, G., GABA-mediated inhibition of locus coeruleus from the dorsomedial rostral medulla, J. Neurosci., 9, 2973, 1989.
- 24 Gallager, D.W. and Aghajanian, G.K., Effect of antipsychotic drugs on the firing of dorsal raphe cells. II. Reversal by picrotoxin, Eur. J. Pharmacol., 39, 357, 1976.
- 25 Gallager, D.W., Benzodiazepines : potentiation of a GABA inhibitory response in the dorsal raphe nucleus, Eur. J. Pharmacol., 49, 133, 1978.
- 26 Cherubini, E., North, R. A. and Williams., J.T., Synaptic potentials in rat locus coeruleus neurons, J. Physiol. (London), 406, 431, 1988.
- 27 Williams, J.T., Bobker, D.H. and Harris, G.C., Synaptic potentials in locus coeruleus neurons in brain slices, Prog. Brain Res., 88, 167, 1991.
- 28 Osmanovic, S.S. and Shefner, S.A., g-aminobutyric acid responses in rat locus coeruleus neurons in vitro : a current-clamp and voltage-clamp study, J. Physiol. (London), 421, 151, 1990.
- 29 Pan, Z.Z. and Williams, J.T., GABA- and glutamate-mediated synaptic potentials in rat dorsal raphe neurons in vitro, J. Neurophysiol., 61, 719, 1989.
- 30 Wang, Q.P., Ochiai, H. and Nakai, Y., GABA-ergic innervation of serotonergic neurons in the dorsal raphe nucleus of the rat studied by electron microscopy double immunostaining, Brain Res. Bull., 6, 943, 1992.
- 31 Luque, J.M., Malherbe, P. and Richards, J.G., Localization of GABAA receptor subunit mRNAs in the rat locus coeruleus, Mol.. Brain Res., 24, 219, 1994.
- 32 Zarbin, M.A., Wamsley, J.K. and Kuhar, M.J., Glycine receptors : light microscopic autoradiographic localization with [3H] strychnine, J. Neurosci., 5, 532, 1981.
- 33 Chase, M.H., Soja, P.J. and Morales, F.R., Evidence that glycine mediates the postsynaptic potentials that inhibit lumbar motoneurons during the atonia of active sleep, J. Neurosci., 9, 743, 1989.
- 34 Kaur, S., Saxena, R.N. and Mallick, B.N., GABA in locus coeruleus regulates spontaneous rapid eye movement sleep by acting on GABAA receptors in freely moving rats, Neurosci. Lett., 223, 105, 1997.
- 35 Darracq, L., Gervasoni, D., Soulière, F., Lin, J.S., Fort, P., Chouvet, G. and Luppi, P-H., Effect of strychnine on rat locus coeruleus neurons during sleep and wakefulness, NeuroReport , 8, 351, 1996.
- 36 Chouvet, G., Akaoka, H. and Aston-Jones, G., Serotonin selectively decreases glutamate-induced excitation of locus coeruleus neurons, C.R. Acad. Sci. (Paris), 306, 339, 1988.
- 37 Akaoka, H., Charléty, P.J., Saunier, C.F., Buda, M. and Chouvet, G., Combining in vivo volume-controlled pressure microinjection with extracellular unit recording, J. Neurosci. Meth., 42, 119, 1992.
- 38 Maloney, K.J., Cape, E.G., Gotman, J. and Jones, B.E., High-frequency g- encephalogram activity in association with sleep-wake states and spontaneous behaviors in the rat, Neuroscience, 76, 541, 1997.
- 39 Gervasoni, D., Darracq, L., Fort P., Soulière F., Chouvet, G and Luppi, P.-H., Electrophysiological evidence that noradrenergic neurones of the rat locus coeruleus are tonically inhibited by GABA during sleep. Eur. J. Neurosci., 10, ,1998.
- 40 Chiang, C. and Aston-Jones, G., A 5-Hydroxytryptamine2 agonist augments g-aminobutryric acid and excitatory amino acid inputs to noradrenergic locus coeruleus neurons, Neuroscience, 54, 409, 1993.
- 41 Blume, H.W., Pittman, Q.J. and Renaud, L.P., Sensitivity of identified hypothalamic neurons to GABA, glycine and related amino acids; influence of bicuculline, picrotoxin and strychnine on synaptic inhibition, Brain Res., 209, 145, 1981.
- 42 Murphy, P.C. and Sillito, A.M., The binocular input to cells in the feline dorsal lateral geniculate nucleus (dLGN), J. Physiol. (London), 415, 393, 1989.
- 43 Peng, Y.B., Lin, Q. and Willis, W.D. Effects of GABA and glycine receptor antagonists on the activity and PAG-induced inhibition of rat dorsal horn neurons, Brain Res., 736, 189, 1996.
- 44 Bennett, J.A., McWilliam, P.N. and Shepheard, S.L., A gamma-aminobutyric-acid-mediated inhibition of neurons in the nucleus tractus solitarius of the cat, J. Physiol. (London), 392, 417, 1987.
- 45 Champagnat, J., Denavit-Saubié, M., Moyanova, S. and Rondouin, G. Involvement of amino acids in periodic inhibitions of bulbar respiratory neurons, Brain Res., 237, 351, 1982.
- 46 Jordan, D., Mifflin, S.W. and Spyer, K.M., Hypothalamic inhibition of neurons in the nucleus tractus solitarius of the cat is GABA mediated, J. Physiol. (London), 399, 389, 1988.
- 47 Palombi, P.S. and Caspary, D.M., GABAA receptor antagonist bicuculline alters response properties of posteroventral cochlear nucleus neurons, J. Neurophysiol., 67, 738, 1992.
- 48 Curtis, D.R. and Johnston, G.A.R., Amino acid transmitters in the mammalian central nervous system, Ergebn. Physiol., 69, 79, 1974.
- 49 Curtis, D.R., Duggan, A.W., Felix, D. and Johnston, G.A.R., Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat, Brain Res., 32, 69, 1971.
- 50 Otis, T.S., Staley, K.S., Mody, I., Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release, Brain Res., 545, 142, 1991.
- 51 Pitler, T.A. and Alger, B.E., Postsynaptic firing reduces synaptic GABAA responses in hippocampal pyramidal cells, J. Neurosci., 12, 4122, 1992.
- 52 Sun, M-K. and Guyenet, P.G., GABA-mediated baroreceptor inhibition of reticulo-spinal neurons, Am. J. Physiol., 249, 672, 1985.
- 53 McGehee, D.S. and Role, L.W., Presynaptic ionotropic receptors, Curr. Opin. Neurobiol., 6, 342, 1996.
- 54 Luppi, P-H., Fort, P., and Jouvet, M., Iontophoretic application of unconjugated cholera toxin B subunit CTb combined with immunohistochemistry of neurochemical substances : a method for transmitter identification of retrogradely labeled neurons, Brain Res., 534, 209, 1990.
- 55 Luppi, P.H., Aston-Jones, G., Akaoka, H., Chouvet, G. and Jouvet, M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin, Neuroscience, 65, 119, 1995.
- 56 Peyron, C., Luppi, P.-H., Fort, P., Rampon, C. and Jouvet, M., Lower brainstem catecholamine afferents to the rat dorsal raphe nucleus, J. Comp. Neurol., 364, 402, 1996.
- 57 Cedarbaum, J.M. and Aghajanian, G.K., Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique, J.Comp.Neurol., 178, 1, 1978.
- 58 Rampon, C., Peyron, C., Gervasoni, D., Cespuglio, R., Fort, P. and Luppi, P-H., Localization of the glycinergic neurons projecting to the rat locus coeruleus, dorsal raphe and trigeminal motor nuclei, Soc. Neurosci. Abstr., 22, 1838,1996.
- 59 Peyron, C., Luppi, P-H., Rampon, C. and Jouvet, M., Location of the GABA-ergic neurons projecting to the dorsal raphe nucleus and the locus coeruleus of the rat, Soc. Neurosci. Abstr., 21, 373, 1995.
- 60 Aghajanian, G.K. and Wang, R.Y., Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique, Brain Res., 238, 463, 1977.
- 61 Stamp, J.A., Semba K., Extent of colocalization of serotonin and GABA in the neurons of the rat raphe nuclei, Brain Res., 677, 39, 1995.
- 62 Sastre, J.P., Buda, C., Kitahama, K. and Jouvet, M., Importance of the ventrolateral region of the periaqueductal gray and adjacent tegmentum in the control of paradoxical sleep as studied by muscimol microinjection in the cat, Neuroscience, 74, 415, 1996.
- 63 Beitz, A., The Rat Nervous System, Second Edition, G. Paxinos (ed), San Diego, 173, 1995.
- 64 Rampon, C., Peyron, C., Petit, J.M., Fort, P., Gervasoni, D.and Luppi, P.H., Origin of the glycinergic innervation of the rat trigeminal motor nucleus, NeuroReport, 7, 3081, 1996.
- 65 Holstege, J.C. and Bongers, C.M.H., A glycinergic projection from the ventromedial lower brainstem to spinal motoneurons. An ultrastructural double labeling study in rat, Brain Res., 566, 308, 1991.
- 66 Yamuy, J. Mancillas, J.R., Morales, F.R. and Chase, M.H., C-Fos expression in the pons and medulla of the cat during carbachol-induced active sleep, J. Neurosci., 13, 2703, 1993.
- 67 Asala, S.A., Okano, Y., Honda, K., Inoue, S., Effects of medial preoptic area lesions on sleep and wakefulness in unrestrained rats, Neurosci. Lett., 114, 300, 1990.
- 68 Lucas, E.A. and Sterman, M.B., Effect of a forebrain lesion on the polycyclic sleep wake patterns in the cat, Exp. Neurol., 46, 368, 1975.
- 69 McGinty, D.J and Stermann, M.B., Sleep suppression after basal forebrain lesions in the cat, Science, 160, 1253, 1968.
- 70 Sallanon, M., Denoyer, M., Kitahama, K., Aubert, C., Gay, N. and Jouvet, M., Long lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat, Neuroscience, 32, 669, 1989.
- 71 John, J., Kumar, V.M., Gopinath, G., Ramesh, V., Mallick, H., Changes in sleep-wakefulness after kainic acid lesion of the preoptic area in rats, Jpn. J. Physiol., 44, 231, 1994.
- 72 Sterman, M.B. and Clemente, C.D., Forebrain inhibitory mechanisms, sleep pattern induced by basal forebrain stimulation in behaving cat, Exp. Neurol., 6, 103, 1962.
- 73 Kaitin, K.I., Preoptic area unit activity during sleep and wakefulness in the cat, Exp. Neurol., 83, 347, 1984.
- 74 Szymusiak, R. and McGinty, D.J., Sleep-related discharge in the basal forebrain of cats, Brain Res., 370, 82, 1986.
- 75 Koyama, Y. and Hayaishi, O., Firing of neurons in the preoptic/anterior hypothalamic areas in rat : its possible involvement in slow wave sleep and paradoxical sleep, Neurosci. Res., 19, 31, 1994.
- 76 Sherin, J.E., Shiromani, P.J., McCarley, R.W., Saper, C.B., Activation of ventrolateral preoptic neurons during sleep, Science, 271, 216, 1996.
- 77 Vanni-Mercier, G., Sakai, K., Salvert, D. and Jouvet, M., Waking-state specific neurons in the caudal hypothalamus of the cat, C.R. Acad. Sci. (Paris), 298, 195, 1984.
- 78 Von Economo, C., Handbuch des Normalen und Patholigischen Physiologie, A. Von Bethe G.V., Bergman G., Embden and U.A. Ellinger (Eds), Berlin, 291, 1926.
- 79 Lin, J-S, Hou, Y., Sakai, K. and Jouvet, M., Histaminergic inputs to the mesopontine tegmentum and their role in the control of cortical activation and wakefulness in the cat, J. Neurosci., 15, 1523, 1996.
- 80 Lin, J.S., Sakai, K., Vanni-Mercier, G. and Jouvet, M., A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjections of muscimol in freely moving cats, Brain Res., 429, 225, 1989.
- 81 Woch, G., Davies, R.O., Pack, A.I. and Kubin, L., Behavior of raphe cells projecting to the dorsomedial medulla during carbachol-induced atonia in the cat, J. Physiol. (London), 490, 745, 1996.
- 82 Jolas, T. and Aghajanian, G.K., Opioids suppress spontaneous and NMDA-induced inhibitory postsynaptic currents in the dorsal raphe nucleus of the rat in vitro, Brain Res., 755, 229, 1997.
- 83 Yamuy, J., Sampagna, S., Lopez-Rodriguez, F., Luppi, P-H., Morales, F.R. and Chase, M.H., Fos and serotonin immunoreactivity in the raphe nuclei of the cat during carbachol-induced active sleep : a double-labeling study, Neuroscience, 67, 211, 1995.
- 84 Maloney K.J. and Jones B.E., C-Fos expression in cholinergic, GABAergic and monoaminergic cell groups during paradoxical sleep deprivation and recovery, Abstr. Soc. Neurosci., 23, 2131, 1997.
- 85 Bird, S.J. and Kuhar, M.J., Iontophoretic applications of opiates to the locus coeruleus, Brain Res., 122, 523, 1977.
- 86 Porkka-Heiskanen, T., Strecker, R.E., Thakkar, M., Bjorkum, A.A., Greene, R.W. and McCarley, R.W., Adenosine : a mediator of the sleep-inducing effects of prolonged wakefulness, Science, 276, 1265, 1997.
- 87 Pan, W.J., Osmanovic, S.S. and Shefner, S.A., Characterization of the adenosine A1 receptor-activated potassium current in rat locus ceruleus neurons, J. Pharmacol. Exp. Ther., 273, 537, 1995.
- 88 Shefner, S.A. and Chiu, T.H., Adenosine inhibits locus coeruleus neurons : an intracellular study in a rat brain slice preparation, Brain Res., 366, 364, 1986.
FIGURES
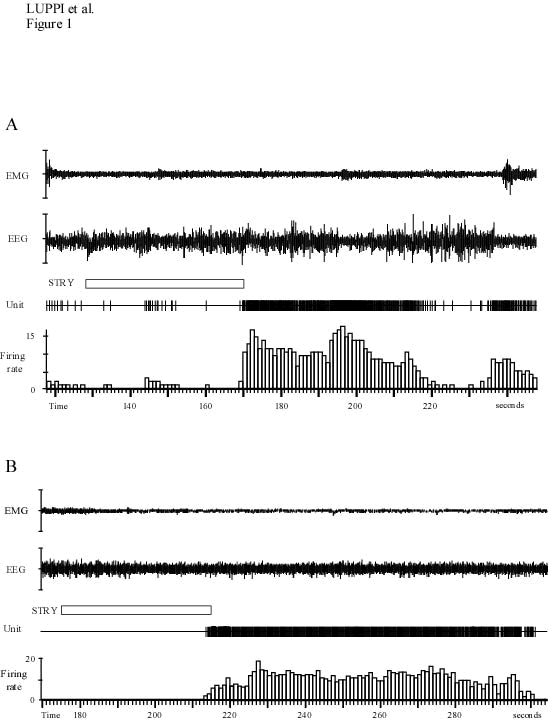
A : Effects of an iontophoretic application of strychnine (90 nA, 42 s). This application induced an activation of the neuron during SWS (the electroencephalogram or EEG with high voltage slow activity and spindles), from 0.16 to 8.9 Hz starting 42 seconds after the onset of the drug application. During the following short period of W (193-200 s), the discharge rate of the neuron reached 14 Hz. Then, with the return of slow waves, it strongly decreased up to the next W phase during which the discharge rate of the neuron increase again above the normal W values.
B : Effect of an iontophoretic application of strychnine during PS (80 nA, 41 s). Note the sustained activation of the LC unit starting about 40s after the onset of the strychnine application.
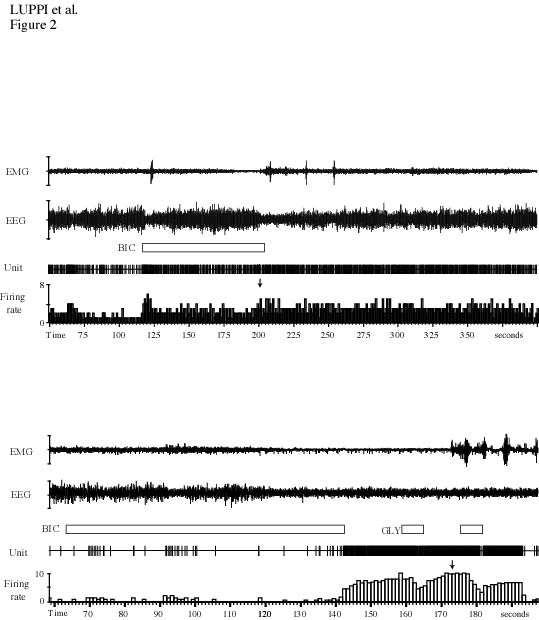
A : Effects of an iontophoretic application of bicuculline (100 nA, 59 s) during SWS. Note the strong increase in the discharge rate of the LC neuron, from 0.3 to 7,8 Hz. The effect appeared 59 seconds after the onset of the ejection. Note that in contrast to strychnine (see figure 2A), during the short period of W, the discharge rate of the neuron did not further increase (arrow).
B : Polygraphic recordings displaying the electromyogram (EMG), EEG, the unit activity of a LC neuron, its firing rate, and the effect of the iontophoresis of bicuculline (50nA, 52s) during PS. GLY still inhibits this LC neuron during the bicuculline-induced activation.
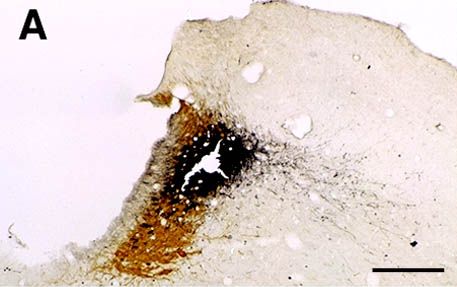
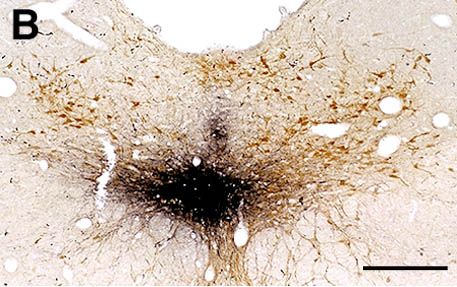
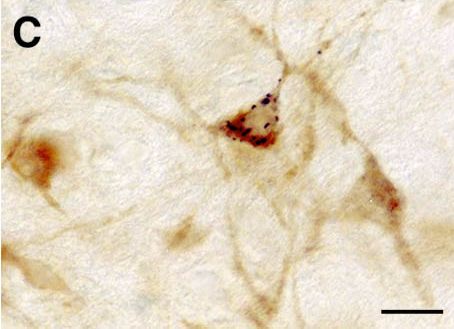
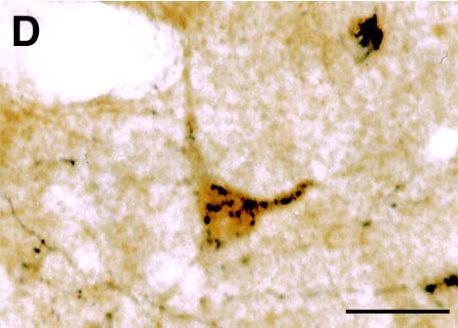
A and B : Photomicrographs illustrating small CTb injection sites limited to the LC (A) or the ventral part of the DRN (B). C : Photomicrograph showing a GAD- (light brown) and CTb double-labeled (black granules) neuron localized in the lateral hypothalamic area after CTb injection in the DRN. D : Photomicrograph illustrating a glycine (light brown) and CTb (black granules) labeled neuron localized in the ventrolateral part of the periaqueductal gray following CTb injection in the DRN. Bars : 250Ám (A-B) and 20Ám (C-D).
 |
|
 |
|
 |
|
 |
Figure 4 : Schematic representation of the glycinergic and GABAergic afferents to the LC, DRN, motor trigeminal nucleus (Mo5) and spinal motoneurons.