| The Brain Histaminergic System and arousal mechanisms |
| by J.S. Lin, M.D. |
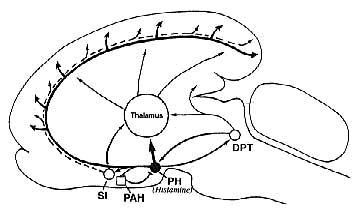
In the mammalian brain, histamine is synthesized from L-histidine by histidine decarboxylase and predominately inactivated by HA-N-methyltransferase. Central actions of histamine are mediated by postsynaptic H1, and H2 receptors, as well as H3 receptors which possess characteristics of both auto- and hetereo-receptors(1). Histaminergic neurons are located almost exclusively in the posterior hypothalamus (Fig. 1), a brain area known for its importance in waking mechanisms since lesions in this area cause hypersomnia. Most histaminergic cells are aggregated in the tuberomammillary nucleus. These cells send ascending and descending axons to diverse brain areas, including the cerebral cortex, thalamus and other structures known for their importance in sleep-wake processes such as the preoptic and anterior hypothalamus, substantia innominata, dorsal pontine tegmentum and raphe dorsalis (2) (Fig. 2). This anatomical organization suggests that the histaminergic system can influence the neuronal excitability and activity of large brain areas.
Presumed histaminergic neurons in vivo discharge tonically and specifically during wakefulness in the cat (3). Increased release of histamine in the posterior hypothalamus of monkeys is observed at moments of awakening and is maintained during each waking episode (4). Electrophysiological studies in vitro reveal that activation of H1 receptors on thalamic relay neurons results in a switch of neuronal discharge from rhythmic burst to single tonic activity and thereby might promote the alternation from slow wave sleep to waking. Activation of H2 receptors, on the other hand, facilitates cortical and hippocampal activity (5). These results suggest that histaminergic cells increase their activity during wakefulness and activate cerebral target areas.
Pharmacological data also support an arousing role of histamine. Various experiments impairing or blocking the transmission of histamine all increase cortical slow activity and deep slow wave sleep, whereas enhancements of histaminergic activity promote wakefulness (6,7). Inactivation of neurons in the posterior hypothalamus, including histaminergic cells, by muscimol (a GABA agonist) induces hypersomnia and hypothermia in normal cats and those rendered insomniac by p-chlorophenylalanine (8), an inhibitor of 5-HT synthesis. Other forms of experimentally-induced insomnia and hyperthermia such as those caused by amphetamine ad ministration or lesions of the preoptic area (9) , are also reversed by muscimol microinjections in the posterior hypothalamus. Inhibition of histamine synthesis in the same area increases slow wave sleep, whereas increasing endogenous histamine by block ade of its degradation elicits long-lasting wakefulness (6). It appears that the posterior hypothalamus plays an important role in arousal through the activity of histaminergic neurons.
The preoptic/anterior hypothalamus, which receives a marked histaminergic innervation, is a forebrain structure involved in sleep generation. Microinjections of histamine in this region induce hyposomnia. This action is probably me diated by H2 receptors, since microinjections of impromidine, an H2 agonist, causes the same effects. In the dorsal pontine tegmentum, where histamine depolarizes cholinergic neurons (10), microinjections of histamine or H1 receptor agonists cause long-lasting cortical desynchronization and episodes of quiet wakefulness, effects at tenuated by a pretreatment with mepryamine, and H1 antagonist.
These results suggest histaminergic neurons in the posteriorhypothalamus constitute one of the major excitatory sources of cortical activation during arousal. Cortical activation and arousal are, therefore, ensured not only by the classically known brainstem-thalamocortical systems and the more studied transmitters such as acetylcholine and catecholamines, but also by histaminergic neurons, including both their ascending and descending pathways (Fig. 2). Although the histaminergic system receives much less attention than the other brain systems, I believe that more active studies in this field will increase our knowledge on the control of sleep and wakefulness and may make useful contributions to Sleep Medicine.
|
|
|
Figure 2 Possible pathways involved in the histaminergic regulation of sleep and wakefulness. During arousal, histaminergic neurons might elicit cortical activation either directly by their cortical projec tions, or indirectly by their activating action on the thalamus, substantia innoninata (Sl) in the basal forebrain or dorsal pontine tegmentum (DPT), as well as by inhibiting sleep generating mechanisms in the preoptic and anterior hypothalamus (PAH). See also text. |
References
- Schwartz, J.C. Arrang, M. Garbarg, H. Pollard, M. Ruat (1991) Physiol. Rev. 71: 1-51.
- Lin, J.S., K. Kitahama, P. Fort, P. Panula, R.M. Denney, M. Jouvet (1993) J. Comp. Neurol. 330: 405-420.
- Sakai, K. M. El Mansari, J.S. Lin, Z.G. Zhang, G. Vanni-Mercier (1990) in: Mancia & Marini (eds), The Diencephalon and Sleep, Raven Press, New York.
- Onoe, H.Y. Yamatodani, K. Watanabe, K. Ono, T. Mochizuki, H. Wada, O. Hayaishi (1992) J. Sleep Res. 1: Sup. 1. p 166.
- McCormick, D. (1992) Prog. Neurobiol. 39: 337-388.
- Lin, J.S., K. Sakai, M. Jouvet (1988) Neuropharmacol. 27:111-122.
- Lin, J.S., K. Sakai, G. Vanni Mercier, J.M. Arrang, M. Garbarg, J.C. Schwartz, M. Jouvet (1990) Brain Res. 523:325-330.
- Lin, J.S., K. Sakai, G. Vanni-Mercier, M. Jouvet (1989) Brain Res. 479-225-240.
- Sallanon, M., M. Denoyer, K. Kitahama, C. Aubert, N. Gay, M. Jouvet (1989) Neurosci. 32: 669-683.
- Khateb, A. M. Serafin, M. Muhlethaler (1990) Neurosci. Lett 112- 257-262.