| The states of sleep |
| Michel Jouvet Scientific American (1967) |
Light and deep sleep differ physiologically, deep sleep having much in common with being awake. Studies with cats now suggest that the two states of sleep are induced by different biochemical secretions.
Introduction
Early philosophers recognized that there are two distinctly different levels of sleep. An ancient Hindu tale described three states of mind in man: (1) wakefulness (vaiswanara), in which a person "is conscious only of external objects [and] is the enjoyer of the pleasures of sense"; (2) dreaming sleep (taijasa), in which one "is conscious only of his dreams [and] is the enjoyer of the subtle impressions in the mind of the deeds he has done in the past," and (3) dreamless sleep (prajna), a "blissful" state in which "the veil of unconsciousness envelops his thought and knowledge, and the subtle impressions of his mind apparently vanish."
States 2 and 3 obviously are rather difficult to investigate objectively, and until very recently the phases of sleep remained a subject of vague speculation. Within the past few years, however, studies with the aid of the electroencephalograph have begun to lift the veil. By recording brain waves, eye movements and other activities of the nervous system during the different sleep states neurophysiologists are beginning to identify the specific nervous-system structures involved, and we are now in a position to analyze some of the mechanisms responsible.
Brain Activities in Sleep
Lucretius, that remarkably inquisitive and shrewd observer of nature, surmised that the fidgetings of animals during sleep were linked to dreaming. Some 30 years ago a German investigator, R. Klaue, made a significant discovery with the electroencephalograph. He found that sleep progressed in a characteristic sequence: a period of light sleep, during which the brain cortex produced slow brain waves, followed by a period of deep sleep, in which the cortical activity speeded up. Klaue's report was completely overlooked at the time. In the 1950's, however, Nathaniel Kleitman and his students at the University of Chicago took up this line of investigation. Kleitman and Eugene Aserinsky found (in studies of infants) that periods of "active" sleep, alternating with quiescent periods, were marked by rapid eye movements under the closed lids. Later Kleitman and William C. Dement, in studies of adults, correlated the eye movements with certain brain-wave patterns and definitely linked these activities and patterns to periods of dreaming [see "Patterns of Dreaming," by Nathaniel Kleitman; SCIENTIFIC AMERICAN, November, 1960]. In 1958 Dement showed that cats may have periods of sleep similarly marked by rapid eyes movement and fast cortical activity. He called such periods "activated sleep."
Meanwhile at the University of Lyons François Michel and I had been conducting a series of experiments with cats. In the cat, which spends about two thirds of its time sleeping, the process of falling asleep follows a characteristic course, signaled by easily observable external signs. Typically the animal curls up in a ball with its neck bent. The flexing of the nape of its neck is a clear sign that the muscles there retain some tonus, that is, they are not completely relaxed. In this position the cat lapses into a light sleep from which it is easily awakened.
After about 10 to 20 minutes there I comes a constellation of changes that mark passage over the brink into deep sleep. The cat's neck and back relax their curvature, showing that the muscles have completely lost tonus : they are now altogether slack. At the same time there are bursts of rapid eye movements (eight to 30 movements in each burst) in either the side-to-side or the up-and-down direction, like the movements in visual use of the eyes. Occasionally these eyeball movements behind the closed eyelids are accompanied by a sudden dilation of the pupils, which in the main are tightly constricted during sleep. Along with the eye movements go events involving many other parts of the body : small tremors of muscles at the ends of the extremities, causing rapid flexing of the digits and now and then small scratching motions; very rapid movements of the ears, the whiskers, the tail and the tongue, and an episode of fast and irregular breathing.
It is somewhat startling to realize that all this activity goes on during a period in which the animal's muscular system is totally atonic (lacking in tension). The activities are also the accompaniment of deep sleep, as is indicated by the fact that it takes an unusually high level of sound or electrical stimulation to arouse the cat during this phase. The state of deep sleep lasts about six or seven minutes and alternates with periods of lighter sleep that last for an average of about 25 minutes.
To obtain more objective and specific information about events in the brain during sleep we implanted electrodes in the muscles of the neck and in the midbrain of cats. We used animals that were deprived of the brain cortex, since we wished to study the subcortical activities. In the course of extended recordings of the electrical events we were surprised to find that the electrical activity of the neck muscles disappeared completely for regular periods (six minutes long), and the condition persisted when sharp spikes of high voltage showed up now and then in the pontine reticular formation, situated just behind the "arousal center" of the midbrain. These electrical signs were correlated with eye movements of the sleeping animal. Further, we noted that in cats with intact brains both the abolition of muscle tonus and the sharp high-voltage spikes were strikingly correlated with the rapid eye movement and fast cortical activity Dement had described. These findings pre sented a paradox. It was surely strange to find fast cortical activity (generally a sign of wakefulness) coupled with complete muscular atony (invariably a sign of deep sleep) !
The Two Sleep States
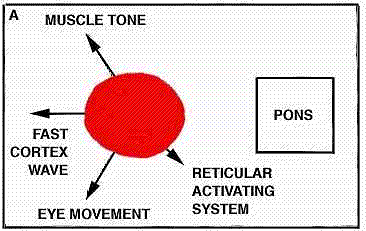
We named this strange state "paradoxical sleep ". It is also called deep sleep, fast-wave sleep, rapid-eye-movement (REM) sleep and dreaming sleep, whereas the lighter sleep that precedes, it is often called slow-wave sleep. We consider paradoxical sleep a qualitatively distinct state, not simply a deepened version of the first stage of sleep. Very schematically (for the cat) we can describe the three states-wakefulness, light sleep and paradoxical sleep in the following physiological terms. Wakefulness is accompanied by fast, low-voltage electrical activity in the cortex and the subcortical structures of the brain and by a significant amount of tonus in the muscular system. The first stage of sleep, or light sleep, is characterized by a slackening of electrical activity in the cortex and subcortical structures, by the occurrence of "spindles," or groups of sharp jumps, in the brain waves and by retention of the muscular tension. Paradoxical sleep presents a more complex picture that we must consider in some detail.
We can classify the phenomena in paradoxical sleep under two heads : tonic (those having to do with continuous phenomena) and phasic (those of a periodic character). The principal tonic phenomena observed in the cat are fast electrical waves (almost like those of wakefulness) in the cortex and subcortical structures, very regular "theta" waves at the level of the hippocampus (a structure running from the front to the rear of the brain) and total disappearance of electrical activity in the muscles of the neck. The principal phasic phenomena are high voltage spikes, isolated or grouped in volleys, that appear at the level of the pons and the rear part of the cortex (which is associated with the visual system). These spikes make their appearance about a minute before the tonic phenomena. Just as the latter show up, the peripheral phasic phenomena come into evidence : rapid eye movements, clawing movements of the paws and soon. The high-voltage spikes during paradoxical sleep in the cat come at a remarkably constant rate: about 60 to 70 per minute.
Our continuous recordings around the clock in a soundproofed cage have shown that cats spend about 35 percent of the time (in the 24-hour day) in the state of wakefulness, 50 percent in light sleep and 15 percent in paradoxical sleep. In most cases the three states follow a regular cycle from wakefulness to light sleep to paradoxical sleep to wakefulness again. An adult cat never goes directly from wakefulness into paradoxical sleep Thus we find that the two states of sleep have well-defined and clearly distinct electrical signatures. Equipped with this information, we are better prepared to search for the nervous structures and mechanisms that are responsible for sleep and dreaming.
The Suppression of Wakefulness
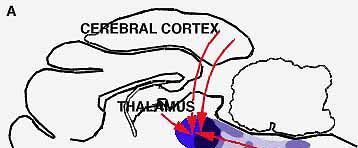
The first and most important question we must answer is this : Does the nervous system possess a specific sleep-producing mechanism ? In other words, should we not rather confine our research to the operations of the mechanism that keeps us awake ? Kleitman has put the issue very clearly ; he observes that to say one falls asleep or is put to sleep is not the same as saying one ceases to stay awake. The first statement implies that an active mechanism suppresses the state of wakefulness-a mechanism analogous to applying the brakes in an automobile, The second statement implies that the wakefulness-producing mechanism simply stops operating a situation analogous to removing the foot from the accelerator. Thus the mechanism responsible for sleep would be negative or passive, not active. Now, it has been known for nearly two decades that the brain contains a center specifically responsible for main aining wakefulness. This was discovered by H. W. Magoun of the U.S. and Giuseppe Moruzzi of Ital.y, working together at Northwestern University [see "The Reticular Formation," by J. D. French; SCIENTIFIC AMERICAN, May, 1957]. They named this center, located in the midbrain, the reticular activating system (RAS). Stimulation of the RAS center in a slumbering animal arouses the animal; conversely, destruction of the center causes the animal to go into a permanent coma. To explain normal sleep, then, we must find out what process or mechanism brings about a deactivation of the RAS for the period of sleep.
On the basis of the known facts about the RAS there seemed at first no need to invoke the idea of a braking mechanism to account for deactivation of the system. The Belgian neurophysiologist Frédéric Bremer suggested that the RAS could simply lapse into quiescence as a result of a decline of stimuli (such as disturbing noise) from the surroundings [see "Sleep," by Nathaniel Kleitman; SCIENTIFIC AMERICAN, November, 1952].
Several years ago, however, explorations of the brain by the Swiss neurophysiologist W. R. Hess and others began to produce indications that the brain might contain centers that could suppress the activity of the RAS. In these experiments, conducted with cats, the cats fell asleep after electrical stimulation of various regions in the thalamus and elsewhere or after the injection of chemicals into the cerebrum. Interesting as these findings were, they were not very convincing on the question at issue. After all, since a cat normally sleeps about two-thirds of the time anyway, how could one be sure that the applied treatments acted through specific sleep inducing centers ? Moreover, the experiments seemed to implicate nearly all the nerve structures surrounding the RAS, from the cerebral cortex all the way down to the spinal cord, as being capable of inducing sleep. It was implausible that a sleep-inducing system could be so diffuse. Nevertheless, in spite of all these doubts, the experiments at least pointed to the possibility that the RAS might be influenced by other brain centers. Moruzzi and his group in Ital.y proceeded to more definitive experiments. Seeking to pin down the location of a center capable of opposing the action of the RAS, they focused their search on the lower part of the brainstem. They chose a site at the middle of the pons in front of the trigeminal nerve, and with cats as subjects they cut completely through the brainstem at that point. The outcome of this operation was that the cats became insomniac : they slept only 20 percent of the time instead of 65 per cent ! The brain cortex showed the characteristic electrical activity of wakefulness (fast, low-voltage activity), and the eye movements also were those of a wakeful animal pursuing moving objects. The experiments left no doubt that the cut had disconnected the RAS from some structure in the lower part of the brainstem that normally exercised control over the waking center. It was as if a brake had been removed, so that the RAS was essentially unrestricted and kept the animal awake most of the time. The new evidence leads, therefore, to the conclusion that sleeping is subject to both active and passive controls. The active type of control consists in the application of a brake on the RAS by some other brain structure or structures; the passive type corresponds to a letup on the accelerator in the RAS itself.
Sleep Centers
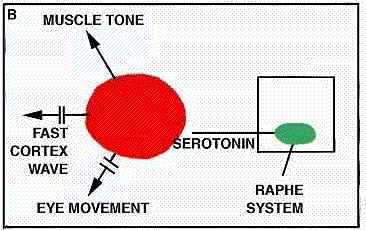
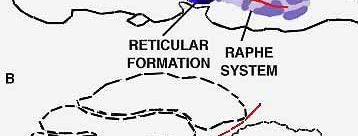
What, and where, are the sleep-inducing centers that act on the RAS ? Our suspicions are now focused on a collection of nerve cells at the midline of the brainstem that are known as the "nuclei of raphe" (from a Greek word meaning "seam" and signifying the juncture of the two halves of the brain). In Sweden, Annica Dahlstrom and Kzell Fuxe have shown that under ultraviolet light these cells emit a yellow fluorescence that shows they are rich in the hormone-like substance serotonin, which is known to have a wide spectrum of powerful effects on the brain and other organs of the body [see "Serotonin," by Irvine H. Page; SCIENTIFIC AMERICAN, December, 1957]. Suspecting from various preliminary pharmacological experiments that serotonin might play a role in sleep, we decided to test the effects of destroying the raphe cells, which are the principal source of the serotonin supply in the brain. We found that when we destroyed 80 percent of these cells at the level of the medulla in cats (the animals could not have survived destruction of a larger percentage), the cats became even more sleepless than those on which Moruzzi had performed his operation. In more than 100 hours of continuous observation with electrical recording instruments, our animals slept less than 10 percent of the time. Our results were closely related to those of Moruzzl's. His operation dividing the brainstem cut through the raphe system. We found that when we destroyed only the raphe cells on one side or the other of the site of his cut, our animals were reduced to the same amount of sleep (20 percent) as those on which he had performed his experiment. This gives us further reason to believe the raphe system may indeed be the main center responsible for bringing on sleep in cats.
These new developments bring serotonin into a prominent place in the research picture and offer an avenue for biochemical attack on the mysteries of sleep. The fact that the raphe cells are chiefly notable for their production of serotonin seems to nominate this substance for an important role in producing the onset of sleep. We have recently been able to demonstrate a significant correlation between the extent of the lesion of the raphe system, the decrease in sleep and the decrease in the amount of serotonin in the brain as measured by means of spectrofluorescent techniques. In physiological terms we can begin to see the outlines of the system of brain structures involved in initiating the on set of sleep and maintaining the first stage of light slumber. At the level of the brainstem, probably within the raphe system, there are structures that apparently counteract the RAS and by their braking action cause the animal to fall asleep. Associated with these structures there presumably are nearby structures that account for the modulations of electrical activity (notably the slow brain waves) that have been observed to accompany light sleep. This slow activity seems to depend primarily, however, on the higher brain structures, particularly the cortex and the thalamus; in a decorticated animal the pattern characteristic of light sleep does not make its appearance. We must therefore conclude that the set of mechanisms brought into play during the process of falling asleep is a complicated one and that a number of steps in the process still remain to be discovered.
Paradoxical Sleep
In searching for the structures involved in paradoxical, or deep, sleep we are in a somewhat better position. When an animal is in that state, we have as clues to guide us not only the electrical activities in the brain but also conclusive and readily observable signs such as the disappearance of tonus in the muscles of the neck. This is the single most reliable mark of paradoxical sleep. Furthermore, it enables us to study animals that have been subjected to drastic operations we cannot use in the study of light sleep because they obliterate the electrical activities that identify the falling-asleep stage.
A cat whose brainstem has been cut through at the level of the pons, so that essentially all the upper part of the brain has been removed, still exhibits the cycle of waking and deep sleep. Such an animal can be kept alive for several months, and with the regularity of a biological clock it oscillates between wakefulness and the state of paradoxical sleep, in which it spends only about 10 percent of the time. This state is signaled, as in normal animals, by the typical slackness of the neck muscles, by the electroencephalographic spikes denoting electrical activity in the pons structures and by lateral movements of the eyeballs.
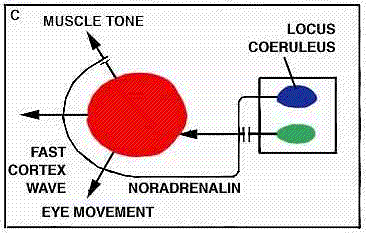
When, however, we sever the brain stem at a lower level, in the lower part of the pons just ahead of the medulla, the animal no longer falls into paradoxical sleep. The sign that marks this cyc]ical state-periodic loss of muscle tonus-disappears. It seems, therefore, that the onset of paradoxical sleep must be triggered by the action of structures somewhere in the middle portion of the pons. Further experiments have made it possible for us to locate these structures rather precisely. We have found that paradoxical sleep can be abolished by destroying certain nerve cells in a dorsal area of the pons known as the locus coeruleus. Dahlstrom and Fuxe have shown that these cells have a green fluorescence under ultraviolet light and that they contain noradrenalin. Hence it seems that noradrenalin may play a role in producing paradoxical sleep similar to the one serotonin apparently plays in bringing about light sleep.
What mechanism is responsible for the elimination of muscular tonus that accompanies paradoxical sleep ? It seems most likely that the source of this inhibition lies in the spinal cord, and Moruzzi and his colleague Ottavio Pompeiano are making a detailed investigation of this hypothesis.
The objective information about paradoxical sleep developed so far gives us some suggestions about the mechanisms involved in dreaming. The controlling structures apparently are located in the dorsal part of the pons. They give rise to spontaneous excitations that travel mainly to the brain's visual tracts, and it seems possible that this excitation is related to the formation of the images that one "sees" in dreams. Regardless of how strongly the brain is stimulated by these spontaneous impulses (as Edward V. Evarts of the National Institute of Mental Health and others have shown by means of microelectrode recordings of the visual system), during sleep the body's motor system remains inactive because a potent braking mechanism blocks electrical excitation of the motor nerves. This inhibitory mechanism seems to be controlled by the hormone-secreting nerves of the locus coeruleus structure. If this structure is destroyed, the animal may periodically exhibit a spasm of active behavior, which looks very much as if it is generated by the hallucinations of a dream. In such episodes the cat, although it evinces the unmistakable signs of deep sleep and does not respond to external stimuli, will sometimes perform bodily movements of rage, fear or pursuit for a minute or two. The sleeping animal's behavior may even be so fierce as to make the experimenter recoil.
All in all the experimental evidence from mammals obliges us to conclude that sleep has a fundamental duality; deep sleep is distinctly different from light sleep, and the duality is founded on physiological mechanisms and probably on biochemical ones as well. Can we shed further light on the subject by examining animal evolution ?
The Evolution of Sleep
Looking into this question systematically in our laboratory, we failed to find any evidence of paradoxical sleep in the tortoise and concluded that probably reptiles in general were capable only of light sleep. Among birds, however, we start to see a beginning of paradoxical sleep, albeit very brief. In our subjects- pigeons, chicks and other fowl- this state of sleep lasts no longer than 15 seconds at a time and makes up only 0.5 percent of the total sleeping time, contrasted with the higher mammals 20 to 30 per cent. In the mammalian order all the animals that have been studied, from the mouse to the chimpanzee, spend a substantial portion of their sleeping time in paradoxical sleep. We find a fairly strong indication that the hunting species (man, the cat, the dog) enjoy more deep sleep than the hunted (rabbits, ruminants). In our tests the former average 20 percent of total sleep time in paradoxical sleep, whereas the latter average only 5 to 10 percent. Further studies are needed, however, to determine if what we found in our caged animals is also true of their sleep in their natural environments.
The evolutionary evidence shows, then, that the early vertebrates slept only lightly and deep sleep came as a rather late development in animal evolution. Curiously, however, it turns out that the opposite is true in the development of a young individual; in this case ontogeny does not follow phylogeny. In the mammals (cat or man) light sleep does not occur until the nervous system has acquired a certain amount of maturity. A newborn kitten in its first days of life spends half of its time in the waking state and half in paradoxical sleep, going directly from one state into the other, whereas in the adult cat there is almost invariably a transitional period of light sleep. By the end of the first month the kitten's time is divided equally among wakefulness, light sleep and paradoxical sleep (that is, a third in each); thereafter both wakefulness and light sleep increase until adulthood stabilizes the proportions of the three states at 35, 50 and 15 per cent respectively.
Considering these facts of evolution and development, we are confronted with the question: What function does paradoxical sleep serve after all? As Kleitman reported in his article "Patterns of Dreaming," Dement found that when he repeatedly interrupted people's dreams by waking them, this had the effect of making them dream more during their subsequent sleep periods. These results indicated that dreaming fulfills some genuine need. What that need may be remains a mystery. Dement's subjects showed no detectable disturbances of any importance - emotional or physiological - as a result of their deprivation of dreaming.
We have found much the same thing to be true of the deprivation of paradoxical sleep in cats. For such a test we place a cat on a small pedestal in a pool of water with the pedestal barely topping the water surface. Each time the cat drops off into paradoxical sleep the relaxation of its neck muscles causes its head to droop into the water and this wakes the animal up. Cats that have been deprived of paradoxical sleep in this way for several weeks show no profound disturbances, aside from a modest speeding up of the heart rate. They do, however, have a characteristic pattern of aftereffects with respect to paradoxical sleep. For several days following their removal from the pedestal they spend much more than the usual amount of time (up to 60 percent) in paradoxical sleep, as if to catch up. After this rebound they gradually recover the normal rhythm (15 percent in deep sleep), and only then does the heart slow to the normal rate. The recovery period depends on the length of the deprivation period: a cat that has gone without paradoxical sleep for 20 days takes about 10 days to return to normal.
The Chemistry of Sleep
All of this suggests that some chemical process takes place during the recovery period. Let us suppose that the deprivation of paradoxical sleep causes a certain substance related to the nervous system to accumulate. The excess of paradoxical sleep during the recovery period will then be occupied with elimination of this "substance," presumably through the agency of "enzymatic" factors that act only during paradoxical sleep.
There is reason to believe that certain enzymes called monoamine oxidases, which oxidize substances having a single amine group, play a crucial role in bringing about the transition from light sleep to paradoxical sleep. We have found that drugs capable of inhibiting these enzymes can suppress paradoxical sleep in cats without affecting either light sleep or wakefulness. A single injection of the drug nialamide, for example, will eliminate paradoxical sleep from the cycle for a period of hundreds of hours. We have also found that this potent drug can suppress paradoxical sleep in cats that have first been deprived of such sleep for a long period in the pool experiment.
The findings concerning the probable importance of the monoamine oxidases in the sleep mechanism raise the hope that it may soon be possible to build a bridge between neurophysiology and biochemistry in the investigation of sleep. If it is indeed a fact that these enzymes play an important role in sleep, this tends to strengthen the hypothesis that serotonin and noradrenalin, which are monoamines, are involved in the two states of sleep-serotonin in light sleep and noradrenalin in paradoxical sleep. There are other bits of chemical evidence that support the same view. For example, the drug reserpine, which is known to prevent the accumulation of monoamines at places where these compounds are usually deposited, has been found to be capable of producing some specific electrical signs of paradoxical sleep in experimental animals. Further, the injection of certain precursors involved in the synthesis of serotonin in the brain can produce a state resembling light sleep, whereas drugs that selectively depress the serotonin level in the brain produce a state of permanent wakefulness.
We can put together a tentative working hypothesis about the brain mechanisms that control sleep. It seems that, the raphe system is the seat responsible for the onset of light sleep, and that it operates through the secretion of serotonin. Similarly, the locus coeruleus harbors the system responsible for producing deep sleep, and this uses noradrenalin as its agent. In cyclic fashion these two systems apply brakes to the reticular activating system responsible for wakefulness and also influence all the other nerve systems in the brain, notably those involved in dreaming.
Dreaming itself, particularly the question of its evolutionary origin and what function it serves, is still one of the great mysteries of biology. With the discovery of its objective accompaniments and the intriguing phenomenon of paradoxical sleep, however, it seems that we have set foot on a new continent that holds promise of exciting explorations.
Figure 1
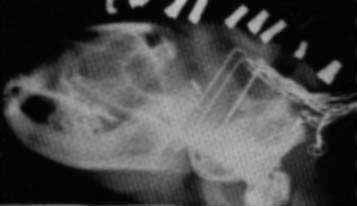
X RAY OF CAT'S HEAD shows a cluster of electrodes with which the author obtained a record of the electrical signal from various parts of the cat's brain. The cat's mouth is at the left; one electrode at far right measures the changes in the animal's neck muscle tension.
Figure 2
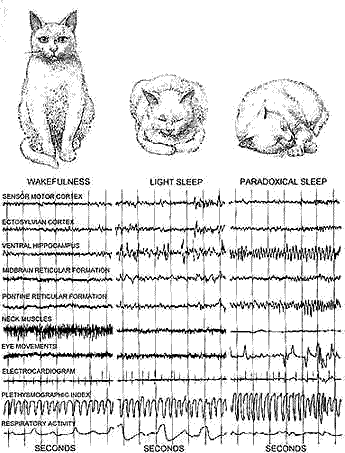
CHARACTERISTIC RHYTHMS associated with deep sleep in a cat (group of traces at right) are 90 much like those of wakefulnes6 (left group) and 6C different from those of light sleep (middle group) that the author has applied the term "paradoxical" to deep sleep. Normal cats spend about two-thirds of the time sleeping. They u6ually begin each sleep period with 25 minutes of light sleep, followed by 6ix or seven minutes of paradoxical sleep. In the latter state they are hard to wake and their muscle6 are relaxed.
Figure 3

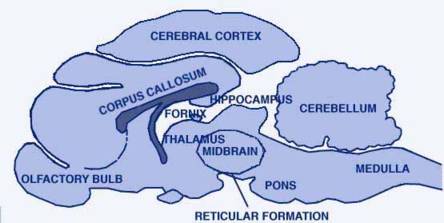
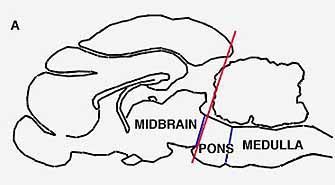
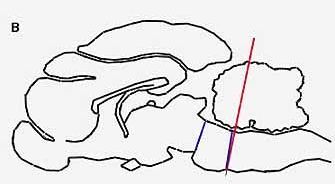
CAT'S BRAIN, seen in front-to-back section, has a number of segments. Some of the principal ones are identified in the illustration "BRAIN SEGMENT". Many segments of the cat's brain such as the cerebellum (top right), have no role to play in sleep.
Figure 4

VARYING RHYTHMS are identified with the various states of sleep. From left to right, a wakeful cat (I ) shows high speed alternations in electric potential in both cortical and subcortical regions of the brain, as well as neck-muscle tension. In light sleep (2) the cat shows a slower rhythm in the traces from the cortical and sub cortical regions, but neck-muscle tension continues. The phasic, or periodic, aspects of paradoxical sleep (3) are marked by isolated spike discharges from the rear of the cortex and the pons, as well as by rapid eye movement and limb movements. Loss of neck-muscle tension is a tonic (4) rather than a phasic phenomenon. Other tonic, or continuous, aspects of paradoxical sleep are high-speed cortical rhythms and regular "theta" waves from hippocampus.
Figure 5
BRAIN SEGMENTS associated with sleep include the reticular formation, which controls wakefulness. This region is under the control of an area in the lower brain. When the control is blocked by making a cut through the pons, a normal cat becomes insomniac.
Figure 6
WORKING HYPOTHESIS,
proposed by the author to provide a bridge between the
neurophysiology and the biochemistry of sleep, suggests that the normal
state of wakefulness (a) is transformed into light sleep (b) when a secretion
produced by the nuclei of raphe modifies many effects of the reticular
activating system. Paradoxical sleep follows (c) when a second secretion,
produced by the locus coeruleus, supplants the raphe secretion and produces
effects that resemble normal wakefulness except for the loss of muscle
tension.
Figure 7
 |
|
 |
|
 |
BRAIN STRUCTURES involved in light sleep include the raphe system, which, by producing the monoamine serotonin, serves to counteract the alerting effects of the brain's reticular formation ("a," color at left). The author suggests that other nearby structures act to modulate the fast wave pattern of the alert cortex into the slower pattern typical of light sleep. Such slow activity, however, is known to depend on higher as well as lower brain structures (b); when a cat is deprived of its cerebral cortex and thalamus, the wave pattern characteristic of light sleep disappears. The reason for this is not yet understood.
Figure 8
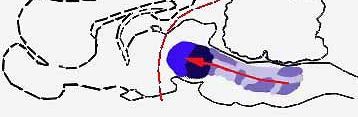
 |
|
 |
PARADOXICAL SLEEP STRUCTURES evidently lie far back along the brainstem. A cat deprived of all its higher brain function by means of a cut through the pons (a) will live for months, alternately awake and in paradoxical sleep. If a cut is made lower (b) along the brainstem, however, the cat will no longer fall into paradoxical sleep, because the cut destroys the brain cells in that region, which produce another monoamine, noradrenalin.
Figure 9
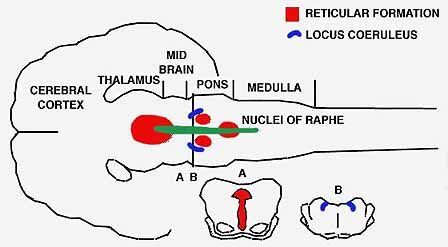
CAT'S BRAINSTEM
is the site of the two groups of cells that produce the substances affecting light and paradoxical sleep. The nuclei of raphe (color) secrete serotonin; nerve cells lower down the pons, known as the locus coeruleus (gray), secrete noradrenalin.
Figure 10
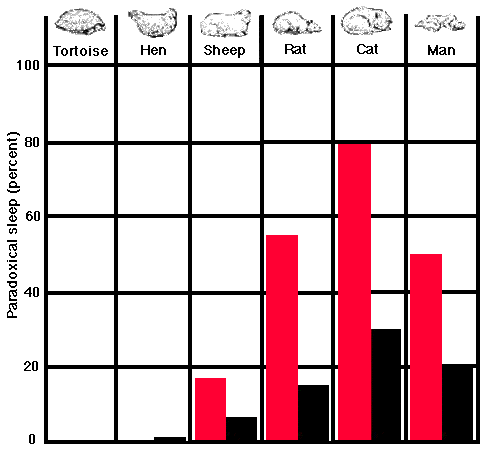
PARADOXICAL SLEEP
among three vertebrate classes of increasing evolutionary complexity is shown as a percentage of each animal's time spent in light sleep. None is known in the case of the reptile, a tortoise; in the case of the hen it is only two-tenths of 1 percent of the total. In the case of each of the four mammal species shown, the newborn spend at least twice as much time in paradoxical sleep (color) as do their adult counterparts (black).