1 Introduction
As long as we do not know how and why sleep forces on us a necessary and recurrent change in the process of our relations with our environment, it is impossible to give a definition of sleep that would satisfy everybody. Indeed the causes and mechanisms of sleep remain unknown despite a great amount of work. Kleitman's huge book (277) itself includes 4,377 references, and yet the latest achievements in the neurophysiology of sleep have only limited coverage. Since it is thus out of the question to undertake a general survey of the physiology of sleep, this paper is limited to a review of some particular aspects of the neurophysiology of sleep in the light of results achieved in the last 5 or 6 years. On the one hand, we must assume that our brain, like our kidneys and heart but unlike our muscular system, does not rest during sleep. On the contrary, it undergoes an active reorganization rather than a real inhibition, and so sleep seems to be an active phenomenon. On the other hand, it appears that behavioral sleep does not proceed from a single process but is the manifestation of two different states of nervous activity, though these are closely interconnected. The electrical brain activity of a sleeping mammal has a recurring evolution proceeding from two opposite modes. The first mode, which is the earliest known (and which is called slow sleep), manifests itself in the presence of a synchronized cortical activity of spindles and/or of high-voltage slow waves. The other mode reveals itself by a low-voltage fast cortical activity similar to arousal activity [activated sleep (122) or paradoxical or rhombencephalic sleep tPS) (254)]. Though it has not yet been proved that these two electric aspects of sleep are the manifestation of a single hypnogenic mechanism or the manifestation of two fundamentally different states, I shall set forth their behavioral and electric aspects successively and then discuss the mechanism of their appearance the last chapter is devoted to their respective interrelations in the light of their phylo- and ontogenetic evolution. I shall not deal in detail with the problems of vegetative, respiratory, digestive, blood, or metabolic (267) variations during sleep for they are clearly stated by Kleitman (277); this study is also limited to the nervous theories of sleep state, though humoral theories are again of topical interest (279, 319, 320).
2 Definitions and Abbreviations
The confused terminology of the states of sleep is indicative of today's increasing interest about the sleep mechanisms. Until we get a biochemical definition of the states of sleep, the terminology must be understood by physiologists, clinicians, zoologists, and even psychologists ! So we must compare the states of sleep at various ages (from newborn infants to old people), in different species (from bird to man), and in various expetimental preparations (normal or pontine animals). That is why we cannot use a terminology based only on the electroencephalogram (EEG). "Slow or synchronized sleep," a term in general use, does not apply to stage I (drowsiness) in man, nor to newborn animals whose EEG does not vary during the different states of vigilance, nor to decorticate animals. The same remark is true for "fast or desynchronized sleep." Calling it "paradoxical sleep" implies no mechanism but expresses the astonishment of the physiologist at the accumulation of EEG and behavioral phenomena diverging from the "orthodox" conception of sleep. If we assume the rhombencephalon is necessary and even sufficient for the occurrence of paradoxical sleep, the term "rhombencephalic sleep" is then justified. The use of "activated sleep" indicates the existence of activating mechanisms that are rather difficult to define. The terms of "light" and "deep sleep" do not apply to man.
The following list, certainly incomplete, gives the terminology used to classify the two states of sleep (in the animal).
1) Synonyms for sleep characterized by slow cortical activity in adult animals sleep (up to 1958); deep sleep (100); light sleep (90); slow wave sleep (SWS) (140); slow sleep, synchronized sleep (240); telencephalic sleep (239); neo-sleep (240); firsl sleep (311); first phase of sleep (380), ortho sleep (420); quiet sleep (in kittens) (86); sleep without jerks (in kittens) (423); non rapid eye movements (NREM) sleep (131).
2) Synonyms for sleep characterized by fast cortical activity and abolition of neck EMG activity in adult animals sonno profondo (161); Tiefen Schlaf (274); activated sleep (122); paradoxical sleep (or paradoxical phase) (254); rhombencephalic phase of sleep (RPS) or rhombencephalic sleep (239); desynchronized sleep (328); fast sleep; fast wave sleep (FWS) (140); deep sleep (90); archeo sleep (240); second sleep (311); second phase of sleep (380); para-sleep (420); oneiric sleep (188) postreaction EEG (in the rabbit) (394); hyperarousal (in the rabbit) (395); phenomene particulier du sommeil (in the rabbit) (150); restless sleep (in kittens) (86); sleep with jerks (in kittens) (423); sommeil sismique (425); rapid eye movemenbs (REM) deep (131).
Nevertheless, recent progress in histochemical methods has permitted us to map out serotonergic and catecholaminergic systems in the brain stem (114, 169). The possible role of these systems in sleep mechanisms is also discussed separately.
It is necessary to make a few remarks concerning methodology; the study of sleep mechanisms is one of the fields of neurophysiology in which methodological problems are of paramount importance. Indeed, there is no absolute behavioral or electroencephalographic criterion of sleep. The fact that the low-voltage fast cortical activity (arousal reaction) (332) and behavioral arousal often occur together has led to an interchangeable but deplorable use of these terms. The electroencephalogram (EEG) variations are often admitted in acute experimentation as a sufficient criterion of wakefulness. Yet in no case does the state of the corticogram allow us to presume whether an animal is asleep or awake. If acute experimentation is carried out on anesthetized animals, the irreversible character of narcosis at once causes us to disregard one of the fundamental criteria of physiological sleep, namely its immediate interruption under the influence of a strong stimulation. The use of animals under curare or of the encephale isole preparation is doubtless a step forward. Yet, the difficulty of obtaining suitable ventilation (1) or of maintaining correct blood pressure without adding drugs hardly allows us to achieve a stable humoral state of long duration. Many humoral factors then can hasten the appearance of slow cortical waves (fall of blood pressure, alkalosis, etc.), whose mechanisms are not necessarily the same as those of the slow waves of sleep. Besides, the behavioral reactions of an animal under curare or encephale isole are much decreased, apart from the intrinsic or extrinsic ocular effectors; and curare may have synchronizing influences, which Hodes (211) ascribed to the reduction in proprioceptive afferent impulses. This reduction in proprioceptive afferent streams would then account for the conspicuous propensity of the encephale isole preparation to a synchronized cortical record (208, 344). Further, the mere fact of the "contention" of an unanesthetized animal not under curare in a stereotaxic apparatus, even if we disregard any painful stimulation (447), involves EEG correlations of sleep (fast spindles, absence of slow waves and of paradoxical sleep) different from those obtained in conditions of complete freedom. The same fact has been noticed after injection of reserpine, which produces EEG synchronization if the injection has been made before surgical manipulations but an arousal record if the injection is made after surgery (411).
This is why only chronic experimentation combining all the present resources of polygraphy is the appropriate means of studying the states of sleep. But this also is beset by many difficulties. The first is technical; it is difficult to record various zones of the brain stem in the same animal at the same time, though new technioues (74, 216) now allow a wider exploration. In addition, the unit exploration and identification of recorded neurons meet important technical difficulties. Intracellular recording, which would be useful in chronic conditions, has not yet been achieved. A second difficulty is that natural sleep is a spontaneous phenomenon (for we are still unaware of most of its triggering mechanisms). Sleep does not always occur when the experimenter wishes it and often occurs when it is not wanted; since Pavlov's time (352) this appearance of sleep in many circumstances has been considered to be within the limits of "internal inhibition." This is particularly true in the cat, which sleeps spontaneously for some 60-70 % of the day. The use of continuous polygraphic recordings (24 hr/ day, 7 days/week) has permitted us to describe very stable circadian sleep patterns in laboratory cats (120, 414) (see Table 1). The high percentage of spontaneous sleep makes very difficult the interpretation of the eventual appearance of sleep during or after any electrical (or chemical) central or peripheral "hypnogenic" stimulation. Consequently results obtained with these methods are dealt with in a special section, since their value is more limited than are the results of experiments of localized section or destruction. Yet even these latter methods do not elude criticism. Is it possible to study the integral phenomenon of sleep with the methods of serial sections used by Sherrington in the course of his analysis of the postural tonus? In the case of total section of the brain stem, which part of the cat is to be studied-the upper or the lower part? (See the remarkable discussion in ref 374.) Can we assume that after a circumscribed nervous lesion of the brain stem early EEG phenomena are the most important, or, on the contrary, are the most important phenomena the responses observed after "recovery" -the suppression of a probable "diaschisis" or the appearance of a possible super sensitivity of denervation (93, 410)?
II. State of sleep chacterized by slow cortical activity slow sleep
A. Behavioral Aspects
This section might seem unnecessary, since anyone can recognize a sleeping cat by its posture (and therefore by the persistence of a certain muscular tonus), by its slow and steady breath, and by the stillness of the eyeballs behind closed eyelids. Yet there is no specific behavioral criterion of slow sleep because the relationship between synchronized or slow cortical activity and sleep behavior is not absolute. Indeed an animal may show spindles or slow cortical waves while standing and showing a waking behavior (l04, 145) or while crouching, lying, or coiled into a ball. However, this dissociation between slow activity and arousal behavior, which often occurs after an atropine injection (438), is normally rather infrequent. But the problem of judging the behavior of the animal is far more difficult in preparations with nervous lesions. Although it is fairly easy to notice the alternation of sleep and arousal in thalamic, hypothalamic, or mesencephalic animals (27, 36, 240, 374, 431, 441), this distinction is less obvious in the posterior mesencephalic or chronic pontine animal in which only two behavioral states appear clearly-a hypertonic state (comparable to arousal) and a state of atony with eye movements (corresponding to PS) (240, 252).
Thus only a few of the behavioral criteria of slow sleep in a normal animal have an absolute value. Ocular signs are useful the myosis [ascribed to a hyper tonus of the neurons of the Edinger-Westphal nucleus (64, 381)] is very marked and the nictitating membranes are relaxed, but these criteria obviously have less value when the peduncular area of the brain stem is destroyed. The study of muscular activity does not yield information of pathognomonic value. There always remains a tonic activity at the level of the neck muscles, which is often but not always lower than in wakefulness (254). A deeper analysis of the motor system does not reveal important shifts at the level of the spinal monosynaptic or polysynaptic reflexes, where amplitude remains the same as in wakefulness (181, 183, 284). The vegetative system does not play an important part in the slow sleep state. There is only a small decrease in blood pressure in this state in the cat compared with the waking state (90, 261). Changes in cardiac and respiratory activity are not sufficient in themselves to confirm the slow sleep state in the cat.
Measurement of the arousal threshold (through stimulation of the mesencephalic tegmentum) is perhaps the best criterion. According to some authors (39, 42, 240) the behavioral arousal threshold rises slightly (30 %) during slow sleep. But others have described a steady and recurrent rise in the reticular arousal threshold in the rat (380) and the rabbit (379) during the phases of slow sleep. The increase reaches its peak during the period preparatory to PS. The study of the arousal threshold in response to auditory stimulation is difficult since habituation to auditory stimuli may occur.
Thus the dissociation that may occur between the cerebral slow activity and behavioral sleep makes the physiologist face an ever-present contradiction (and we cannot avoid it in this review). He will either rely on the EEG criterion alone, thus leading himself to believe in the appearance of sleep in animals showing behavioral arousal with a synchronized activity (104, 159), or he will estimate the criteria of slow sleep without the help of the EEG criterion [as is the case in the chronic pontile animal whose subcortical activity always remains fast (240)]. But such an estimation will be purely subjective-when does arousal and relaxed state end and when does sleep start ?
So the finding of a pathognomonic behavioral criterion of slow sleep good for any type of preparation, as well as the establishment of an electrical subcortical activity (or of a biochemical criterion) that could be the cause or the reflection of this behavioral criterion, remains the major and most urgent aim of the study of the physiology of sleep.
II. State of sleep chacterized by slow cortical activity slow sleep
B. Electrophysiological Aspects
The EEG aspects of slow sleep (see Fig. 1) have been known for a long time (130, 204, 274, 371). They consist of the appearance of 11- to 16-cycles/sec spindles of large amplitude predominant at the level of the frontal and associative areas, whereas the synchronized activity is less important at the level of the auditory and visual areas, the olfactory bulb (154, 200), and the pyriform cortex (162). The spindles are also recorded in bipolar derivation at the level of the mesencephalic reticular formation (RF) (204, 251) and the pyramidal tract (20). They are often, though not always, synchronous with the cortical spindles. They are usually followed by 1- to 4-cycles/sec high-voltage slow waves that are also recorded at the level of the subcortical structures. The spindles and slow waves are of lesser amplitude and may be absent (then a low-voltage fast activity may prevail) at the level of the specific thalamic nuclei (240), the pulvinar (12), and the caudal part of the brain stem. Yet no systematic analysis of the topography of the distribution of slow waves at the level of the subcortical structures has been made by using methods of quantitative integration such as those already used in acute (399) or chronic experiments (23).
Some local peculiarities of brain activity during sleep must be pointed out. At the level of the dorsal or ventral hippocampus and at the level of the structures in efferent relationship with the hippocampus (7), high-voltage spikes (500-900,microv) appear (253). These have also been described during barbiturate narcosis (73, 370). Such an activity still exists at the level of the limbic structures in the animal deprived of the neocortex (240). It is then the only electrical index of the appearance of slow sleep. Thus in the cat only two EEG consecutive aspects, spindles and slow waves, can be recorded during slow sleep. It appears that in carnivora falling asleep there is no stage similar to the first stage of sleep in man (127), during which there is a flattening of alpha activity with a fast activity. A similar short-lasting stage has been described in the chimpanzee (5, 372).
Unitary activity during spontaneous sleep
The mechanisms of sleep would obviously be better known if we could understand the intimate nature of the unit activity shifts at the cortical level, the limbic system, and the reticular formation level; these are the three structures in which, a priori, the most important information may be recorded. In this field chronic experimentation is just beginning and certain interpretations must be borrowed from the results of acute experimentation. Two ways of exploring unit activity have been worked out.
1) The first method investigates the behavior of only one neuron (or of a small group of neurons). In some cases, the shifts of the unit discharges during slow sleep (compared with arousal) are statistically studied, but unfortunately no time correlation is possible with the changes of the EEG within sleep, which lessens the value of the results. Infrequently, the modality of the unit cortical discharges is studied in relation to the two characteristic EEG patterns, spindles and slow waves, and thus more fruitful results are obtained on the influences exerted on the neurons (input).
2) The second method is to study the general activity level of the neurons with techniques of integration. Hence this enables us to learn more of the results of the neuronal activity (output).
Unitary behavior of the neurons during slow sleep.
CORTEX. When the unitary activity is not studied in close temporal relationship with the pattern of sleep EEG, but is compared statistically with the activity of the waking state, the results obtained at the level of the visual (137-139, 215), suprasylvian (143), or auditory (335) cortex show an increase of unit discharges in comparison with waking [which was compared to an increase of noise and so to a decrease of the signal-to-noise ratio (137)]. The statistical analysis of the intervals between unit discharges suggests that a change in the pattern of the discharges occurs rather than a general increase (137). This change in pattern is accompanied by other phenomena supporting a reduction of inhibitory processes the recovery cycle of the visual cortical evoked responses shows a decrease in comparison with waking (144), and the inhibition of spontaneous discharges that are evoked by stimulation of the lateral geniculate nucleus is less important than during waking (141). How ever, the study of the unitary activity of sensory cortices does not always allow us to distinguish between the fiber discharges and the neuron discharges and it might be interesting to know the cortical efferent activity.
Evarts (142) has studied the pyramidal tract (PT) neurons located by antidromic stimulation at the level of the pyramids. During slow sleep the total activity is slightly lower than during waking, while bursts of discharges and silent periods occur in turns. The bursts, which probably but not obviously accompany cortical spindles, are attributed to the "reduction in the effectiveness of a mechanism limiting the frequency of the discharges during waking," and the hypothesis of a disinhibition of some central inhibitory interneurons during sleep has been set forth (142). In this connection it must be pointed out that, in preparations with important lesions of the mesencephalic reticular formation, Martin and Branch (308) have also recorded bursts of unitary activity at the level of PT neurons, and they hypothesize that the reticular lesion suppresses an inhibitory process responsible for the regular discharge of the units during waking. All these data favor existence of a decrease in the inhibitory cortical processes through disinhibition of inhibition during the stage of the spindles. Such unit discharges in bursts had already been described by Adrian and Moruzzi (8) in the spindles of cats under Dial anesthesia. They have also been observed, during physiological sleep, at the level of the lateral geniculate nucleus (216).
The study of the temporal relationship between the different patterns of unit discharges and of general EEG activity is more valuable for it enables us to distinguish two different processes during slow sleep that a total statistical analysis might fail to recognize and might even set aside. The results obtained in acute experiments (18, 111-113, 262) have shown that the gathering in bursts of the units (in sequence when recorded with several microelectrodes) (425, 426) accompanies barbiturate spindles, whereas the slow waves of hypoglycemia may, on the contrary, involve a reduction of discharges (113). Contradictory results nevertheless have been found often (215, 335).
Recently the technique of transcortical bipolar recording, in chronic conditions, revealed a very close correlation between the physiological sleep EEG and the unitary activity of the different generators of the cortex during their stratigraphic analysis (87, 88). Thus the surface-positive or surface-negative spindles are accompanied by bursts of discharges occurring at the level of the superficial dendritic and deep (somato-dendritic) units, whereas surface-negative slow waves (typical of slow sleep) are accompanied by a suppression of the unit activity (due to hyperpolarization of the membrane of neuronal bodies of the deep layers). This recently published study is the only one in which a relationship was found between spindles, slow waves, and cortical unit activity in chronic animals.
RETICULAR FORMATION. Although the reticular formation (RF) activity has been studied in acute conditions (326, 327, 397), the analysis of reticular units in chronic conditions has just started. Strumwasser (416) has described an increase in the unitary activity of the mesencephalic reticular formation during slow sleep contrasting with a surprising reduction of activity during waking. Huttenlocher (223, 224) studied 50 units at the level of the mesencephalic tegmentum during waking and slow sleep. Unfortunately, he did not distinguish between spindles and slow waves, which makes it difficult to interpret his findings. Most of the units of the dorsal part of the mesencephalic tegmentum showed increased activity in bursts during slow sleep, but a small group of units, situated at the level of the ventral part of the tegmentum, showed reduced activity, compared to that during waking. However, Caspers (98, 99) found in the rat that 20% of the mesencephalic reticular units showed a decreasing activity at the beginning of the slowing of the EEG, whereas 80 % showed a complete rest when high-voltage cortical slow waves appeared. On the other hand, at the level of the medulla Caspers (97) found an increased unit activity at the onset of slow sleep. As yet there does not appear to be any study of the relationship between reticular unit activity and slow activity recorded with macroelectrodes at the same level during sleep.
In spite of the large amount of work on the rhinencephalon, there is not a single reference concerning hippocampal unit activity during sleep. Only the amygdala has been studied (393); at this level, the unit activity decreases and shows a tendency to cluster in bursts. The correlation of these bursts with cortical activity has not been studied.
Background activity level. This technique (23) allows us to quantify the high frequency activity recorded with electrodes 20-80 micron in diameter, and it also enables us to evaluate the intensity of the background activity of the neurons (23, 398). Used lately in chronic conditions at the level of the pyramidal tract (pes pedunculi) (20), this method emphasizes that there is a close correlation between the appearance of cortical spindles, the increase of the background level of pyramidal activity, and, on occasion, the existence of bursts of phasic muscular activity in the neck muscles. The level of pyramidal activity, on the contrary, reaches its minimum during the intervals between the spindles. This observation is consonant with that of Adrian and Moruzzi (8) on cats during Dial anesthesia and proves the existence of descending pyramidal volleys during slow sleep. Though this method has not yet been used for other parts of the brain during sleep, it seems of interest to summarize the results of Schlag and Balvin (398, 399) in acute experiments on encephale isole or on the cat under curare. At the level of the motor cortex the results are similar to those obtained by Arduini et al. (20) at the level of the pyramidal tract-reduction of the general level of activity between the spindles and increased activity during the spindles. On the other hand, no one has recorded an increase in the activity level of the mesencephalic reticular formation during spindles; indeed, a decrease in the reticular activity level appeared whenever periods of spindles and cortical slow waves occurred. [On the contrary, the recruiting response elicited by stimulation of the diffuse thalamic system (129, 321) (see 83) involves an increase in the reticular activity level, thus revealing the different natures of the recruiting response and of the spindles of physiological sleep.] So the curve of the background reticular activity level seems to be the opposite of that of the frontal cortex and the pyramidal tract during slow sleep, and during the transition between wakefulness and sleep the background cortical and reticular activity levels decrease in a way parallel to that of waking.
It is too early to synthesize all these results, because most of the investigators unfortunately have made no distinction between spindles and slow waves and also because there are important gaps in our knowledge about the limbic system and the caudal parts of the brain stem. Yet it seems possible to draw a few tentative inferences. During the manifestation of the surface-positive or surface-negative spindles typical when falling asleep, the thalamic origin of which seems unquestionable (see below), the unit activity of superficial and deeper layers of the cortex is subject to a phasic enhancement. This increase of discharge is attributed either to a negative feedback responsible for keeping a certain level of cortical tone (142) or to the manifestation of a disinhibition of cortical inhibitory interneurons ~(still to be demonstrated). Whatever their detailed mechanism, the spindles are accompanied by a phasic increase in the level of background cortical activity at the level of the sensory and motor areas and the pyramidal tract and by a decrease of the background activity level in the midbrain reticular formation. There are no data, however, that allow us to say whether the appearance of these spindles is secondary to the reduction of the reticular activity level or whether it is the actual cause of such a reduction. The decreased background activity of the reticular formation, however, may explain a few peripheral signs of sleep the reduction of muscular tone, in spite of the amount of pyramidal impulses at every spindle, would then appear as a decrease in the tonic activity of the reticular facilitatory descending system (301).
During cortical slow waves, when slow sleep reveals its most typical EEG manifestation, it seems possible to assume that the cortical unitary activity decreases (probably through hyperpolarization at the level of the deeper layers). At this stage too, the reticular activity seems to decrease.
It is undeniably of much interest to know the relationship between the reticular unit activity and the activity recorded with macroelectrodes during sleep, but nevertheless its importance must not be overestimated in attempts to explain the mechanism of sleep. As a matter of fact, in spite of the complete lack of variation of the electrical activity at the brain-stem level (which remains fast and of low voltage), the chronic decorticate animal keeps on presenting short but un deniable episodes of sleep, behaviorally resembling slow sleep. Thus it would be very important to know, in such chronically decorticate animals, what are the shifts of the background activity level of the mesencephalic reticular formation and then to study their possible correlations with the hippocampal unit activity or with that of the brain-stem caudal structures.
Cortical steady potential and other electrical parameters
The appearance of spindles and slow waves of physiological sleep, whether they occur spontaneously or are provoked by phenomena of habituation (389), is accompanied by a shift of the cortical steady potential to the positive side (96, 97, 390, 442), whereas waking is accompanied by a negative shift (22). A reduction of the resistance of superficial layers of the cortex and an increase of the capacitance have been observed during slow sleep by Aladjanova (15). Such changes are attributed to a "moderating influence of the dendritic potentials over the excitability of the neurons through extra-cellular currents." The hypothalamic impedance (reflecting, in a way, the blood flow) decreases, whereas cortical and reticular impedance shows a tendency to increase at the onset of slow sleep, which is attributed to a relaxation of vasomotor tone (50). The temperature of the preoptic area and of the hypothalamus falls by 0.5 C during slow sleep (2, 4).
II. State of sleep chacterized by slow cortical activity slow sleep
C. Structures and Mechanisms Responsible for Slow Sleep
It is difficult to study the mechanisms of sleep without briefly recalling the classical conception of the wakefulness system, which has been the basis for some years of the passive theory of sleep. Later results in some ways explained but also complicated our interpretation of the mechanisms responsible both for fast cortical activity (arousal reaction) and for behavioral wakefulness.
Passive theory activating reticular system and reticular hypothesis of sleep
An outline of the classical conception of the arousal system (163, 164, 30l, 326, 386, 402). Since 1949 (332) it has appeared that the brain-stem RF (see 71) is responsible for cortical arousal through the ascending reticular activating system (ARAS) as well as for behavioral arousal (either through the ARAS and then by a second ary corticofugal effect or by a simultaneous action on the descending reticular facilitatory system) (301, 302). According to the pioneer work of Moruzzi and Magoun (332), the ARAS occupies the brain-stem tegmentum from the medulla to the rostral part of the mesencephalon, and its corticopetal projections take either a thalamic or an extrathalamic route (409). The ARAS, a nonspecific for mation, receives collaterals from various specific pathways (301) as well as corticofugal projections that may converge at the level of the same neurons (70, 165, 325). Having an automatic unit activity (61), the "activating tone" of the ARAS is maintained by sensory inputs as well as by humoral factors. Among the latter, adrenaline would come first (116). The tonic and phasic ascending or descending reticular activity appears to be "catholic" (301). It may, however, be controlled by the cortex via a reticulo-cortico-reticular feedback mechanism (219-221). The hypothesis of a "reticular homeostasis" was recently documented in detail (115, 117). It is not our purpose to discuss this further in this review, for the "time constant" of the damping cortical action over the ARAS (shown in acute experi ment) is too short (a few msec) to explain the phenomena concerning falling asleep.
Passive theory of sleep and reticular hypothesis of sleep. The historical roots of the passive theory of sleep may be traced back quite far (see 330). Kleitman, one of the supporters of this theory, clearly explains its bases "to fall asleep" or "to be unable to remain awake" have not the same meaning. The "first term implies an active onset of sleep, while the other implies a cessation of an active condition of wakefulness" (277), i.e. a passive mechanism. If so, it is not sleep that needs to be explained, but wakefulness. Bremer's experiments (64,66) attributing the "sleep telencephalic syndrome" of the "cerveau isole" preparation to the "suppression, by interruption of the corticopetal paths, of the steady flow of excitatory impulses which are essential for the maintainance of the waking state" (67) advanced a very important electrophysiological argument in favor of Kleitman's concept. Sleep seemed then the result of a specific deafferentation of the telencephalon.
The discovery of the ARAS allowed us to alter this hypothesis slightly without, however, changing its essential meaning. Physiological sleep was then interpreted as the expression of a functional deafferentation of the ARAS "eliminating the waking influence of the ARAS" (332) and so as an absence of wakefulness. This reticular hypothesis of sleep (291) was founded on the extrapolation of the main following results.
Coma resulting from the extensive destruction of the mesencephalic tegmentum (166, 292, 293) or from barbiturate narcosis (whose depressive influence over the ARAS had been shown) (19, 167) was attributed to the interruption of an ascending flow of reticular impulse. Physiological sleep, as compared with coma, was then explained by a functional, passive reduction of the tone of the ARAS. So according to the reticular hypothesis, sleep would be due to the "desactivation en avalanche" of ascending impulses of the ARAS. This deactivation would be initiated by a slowly developing process of neuronal fatigue, precipitated at a given moment by a reduction of sensory input (66, 67).
Numerous criticisms have been raised against the passive theory and the reticular hypothesis of sleep (328).
I) The "desactivation en avalanche" explains neither why sleep may be induced by central or peripheral stimulation (see below), nor why, if it is a question of "neuronal fatigue," sleep may be obtained for such a long time (60 % of the day in the caged cat).
2) The comparison of the coma produced by extensive lesion of the mesencephalic tegmentum with physiological sleep is not allowable. As a matter of fact, in case of coma, the loops between diencephalon and brain stem are interrupted and the extrapyramidal movements that accompany behavioral arousal are no longer possible. On the other hand, it is not certain that a reversible coma tose state (by extensive lesion of the mesencephalic tegmentum in the animal) (3) might not be accompanied by a certain state of "cerebral wakefulness." It is important in this connection to report the anatomoclinical observation of Lhermitte et al. (290) a patient who had a total softening of the mesencephalic reticular formation, and whose behavior was similar to a comatose state (bilateral ptosis and akinetic mutism), was indeed able to answer precisely, through discrete flexion of the forearm, very complicated questions. Thus, this case shows that it is extremely difficult to estimate the "vigilance" level of an animal that is no longer able to manifest behavioral responses after the destruction of the extra pyramidal pathways of its tegmentum. Man alone can use adequate means to express that he can still understand (and hence that he is indeed "awake").
3) The unit exploration of the ARAS has not always revealed a reduction of the unit activity during physiological sleep (224). This argument, however, is not definitive, since there may be a reduction of the general reticular activity level during spindle activity in the animal under curare (399). Nevertheless, such a reduction in activity may be induced by an active mechanism. So the passive theory of sleep does not allow us to explain satisfactorily the processes of falling asleep and gives way now to "active" theories, which are developed below. It is still necessary to examine some recent developments of the physiology of arousal, for other structures than the ARAS may be responsible for cortical or behavioral states of arousal.
Recent aspects of the physiology of wakefulness. Two types of results have recently caused us to modify the general opinion that the mesencephalic reticular formation is the only one responsible for cortical and behavioral waking.
STRUCTURES RESPONSIBLE FOR CORTICAL ACTIVATION (AROUSAL).
The topography of the neurons activating the cerebral cortex (within the ARAS) appears to be more limited than had been first thought, since neither the bulbar RF nor the pontine RF is necessary for the occurrence of a cortical fast low-voltage ac tivity. Indeed, a midpontine section causes an increase of cerebral wakefulness (see below). The most crucial area of the ARAS responsible for cortical arousal seems to belong mainly to the anterior zone of the nucleus reticularis pontis oralis (RPO) and to the posterior zone of the mesencephalic tegmentum, since coagulations only at these levels induce a slow electrical record lasting for several days (29), whereas stimulations at these same levels involve the most tonic ac tivations (62). This region may also be responsible for the cortical activation triggered by cortical stimulation (316) or by ether anesthesia (387), which is well known to be impossible after a brain-stem intercollicular transection (a section anterior to nucleus RPO).
In addition to the ARAS neurons, however, there is another extrareticular system situated in front of the mesencephalon that may be responsible for cortical tonic desynchronization, since the slow cortical activity of the cerveau isole (which is no longer under the influence of the ARAS) yields to "spontaneous" cortical desynchronization, progressively prevalent when the animal survives for more than 8 days (32, 33, 176, 206, 273, 329, 427). The meaning and mechanisms of this desynchronization are still imprecise. It cannot be the manifestation of a telencephalic paradoxical sleep (PS), for the activation is not synchronous with the PS behavioral signs (triggered from the pons) (206). In some cases, this desynchronized cortical activity was even accompanied by ocular signs of wake fulness (tracking ocular movements) (273). The facilitatory role of hypothermia in the occurrence of desynchronization in the cerveau isole first suggests the hypothalamus might be the activating structure, but there is as yet no definite evidence for this point of view (32, 206). It is a matter for chronic, long-lasting experiments, and these observations pose once more the problem of suppression of a possible diaschisis or of a hypersensitivity of denervation whose neurohumoral bases are still unknown.
STRUCTURES RESPONSIBLE FOR BEHAVIORAL AROUSAL.
It is possible, too, to dissociate, by circumscribed lesions, the activating ascending action of the ARAS responsible for cortical arousal and the descending intervention of other structures responsible for behavioral arousal. On the one hand, chronic lesions of the mesencephalic tegmentum may suppress cortical desynchronization after a nociceptive stimulus, though the animal is behaviorally awake (159, 240); on the other hand, limited coagulations of the posterior hypothalamus induce a comatose behavior, without behavioral arousal after nociceptive stimulus, whereas a tonic cortical activation may still occur (159). This corroborates the classical experiments of Ranson (225, 364, 365; see also 338) and the clinical observations of von Economo (135), which suggested that the structures responsible for behavioral arousal were situated at the level of the hypothalamus. It is also at this level that local coolings may provoke the occurrence of sleep behavior (336, 337).
Thus the integrity of the posterior hypothalamus seems to be necessary to waking behavior. The reticular descending system would then appear more like a relay or a servomechanism (128) by comparison with hypothalamic struc tures, since it would be unable by itself to achieve the complex motor integrations of the waking state. This short summary of the evolution of ideas concerning arousal systems allows us to conclude that the ARAS is not the only structure essential to a tonic cortical desynchronization and that the reticular descending system does not itself appear to be sufficient to induce a true behavioral arousal. Moreover, it seems very likely there are structures at the diencephalic level that can themselves induce the occurrence of fast cortical activity, and that the integrity of these structures is necessary for the appearance of a behavioral arousal.
Active theories
Ascending hypothesis.
This theory postulates the existence of synchronizing and sleep-inducing structures in the lower brain stem (327, 328, 331).
PHYSIOLOGICAL EVIDENGE. After a complete midpontine pretrigeminal section (midpontine pretrigeminal preparation MPP (28-3I)) the cortical EEG shows a very definitive predominance of fast activity (78% instead of 37%). Moreover, the oculomotor reactions of this preparation (depending on the IIIrd and IVth nerves) undeniably evoke a true alertness the midpontine cat follows with vertical eye movements any object passing across its visual field (271). There is a mydriatic response to darkness (272) and a pupillary dilatation may occur at presentation of significant visual stimuli (mice) (29), which may be conditioned by hypothalamic stimulation (9,10), whereas visual accommodation persists to near objects. Other experiments allow us to eliminate an eventual irritative action on the ARAS (persistence of alertness for several days), as well as the possible role of certain humoral disturbances (28). Thiopentone injection into the vertebral artery after clamping the basilar artery causes the appearance of a fast cortical activity, whereas injection into the carotid artery is followed by cortical synchronization (298). But a cerveau isole transection, frontal to (or destroying) the anterior part of nucleus RPO, induces a synchronized cortical EEG during the first few days, without there being any ocular sign of wakefulness (10, 11, 29). Since the encephale isole preparation still presents alternate wakefulness and slow sleep (65, 66), it may be concluded that tonically active EEG synchronizing structures, located in the lower brain stem, are able to dampen the arousing activity of the ARAS.
LOCALIZATION OF THESE STRUCTURES. Some experiments favor a bulbar localization.
1) A prebulbar transection induces an increase in the duration of EEG activation, produced by reticular stimulation in the encephale isole prepara tion (56-59).
2) Local coagulation of the solitary tract nucleus area involves increased activity at the level of the short ciliary nerves (responsible for pupillary dilatation), induced after reticular stimulation (59, 63) in the encephale isole preparation.
3) Local reversible cooling of the bulbar floor of the 4th ventricle in the cat encephale isole preparation produces EEG and behavioral arousal, attributed to the inactivation of the bulbar synchronizing structures (whereas cooling of the pontine floor, on the contrary, produces EEG and behavioral sleep signs) (44, 45)
To these results we may add the observation, in the rat, of an increase in unit activity at the solitary tract level at the onset of slow sleep (97). So, there is much evidence (often obtained in acute conditions) suggesting that the tonically active EEG synchronizing structures, which counteract the ARAS tonic activity, are situated at the level of the medulla. However, other experiments, usually chronic, support a posterior pontine localization.
1) A brain-stem hemisection at midpontine level leads to the oc currence of a desynchronized activity at the level of the homolateral hemisphere, and a hemisection made a few millimeters in front induces a synchronized cortical activity; the former is made by suppression of the ascending synchronizing in fluence, the latter by suppression of the ARAS tonic activation. A hemisection situated a few millimeters behind the midpontine section does not cause cortical asymmetry (107, 108) (though logically it should suppress the bulbar ascending synchronizing influences). Rossi et al. (384) agree that retropontine hemisections do not produce any cortical EEG asymmetry and hence attribute an important part in the triggering of cortical synchronization to the posterior zone of the pons. However, subtotal lesions of the posterior part of the pontine RF do not prevent behavioral and EEG slow sleep (240) although they may increase the duration of desynchronized cortical activity in chronic preparations (89).
2) Unilateral chronic lesions of the solitary tract area do not produce cortical asymmetry during sleep (328). Bilateral coagulation of this area does not prevent behavioral sleep (59).
3) Finally, EEG synchronizing structures situated in the spinal cord may exist, since novocaine injection into the spinal cord may itself produce cortical activation (209, 210).
Possible role of serotonergic neurons in slow sleep. Recently, using the new fluorescent techniques of Falck et al. ( 146, 147), Dahlstrom and Fuxe ( 114, 169) have described two systems of monoaminergic neurons in the brain stem. The catecholaminergic neurons (mostly noradrenergic) with green fluorescence are located mostly in 12 groups (A1-A12) in the lateral part of the medulla, pons, and mesencephalon, whereas serotonergic cell bodies with yellow fluorescence (which is increased after injection of a potent inhibitor of mono-amino-oxidase) are almost exclusively located in 9 groups in the raphe nuclei of the brain stem. Serotonergic terminals from these cell bodies have been located in the spinal cord, the brain stem, and the rostral part of the brain.
Destruction of the raphe nuclei of the brain stem in chronic cats leads to a state of almost permanent wakefulness (244), and there is a decrease of 80-90 % in both slow sleep and paradoxical sleep during the first 2 weeks after the operation. A state of permanent EEG and behavioral wakefulness is obtained during the first 4 or 5 days. Destruction of either the anterior or the posterior half of the raphe system decreases slow sleep but less (50-60 %) than does total destruction. These experiments, together with the results of neuropharmacological alterations of brain monoamines on sleep (see below), have led to the hypothesis that "monoaminergic" neurons could be involved in sleep mechanisms.
There are thus many concordant experimental data in favor of synchronizing and sleep-inducing structures in the lower brain stem. Nevertheless more experi ments are needed in order to delineate precisely these structures and to determine whether they belong to some specific nuclei located in the medulla or the pons or whether they belong to a monoaminergic system composed of serotonergic neurons occupying the raphe nuclei from the medulla caudally to the caudal mesencephalon rostrally.
MODE OF ACTION OF EEG SYNCHRONIZING CAUDAL STRUCTURES. The mode of action of these structures, located either at the level of the posterior part of the pons or at the level of the medulla, is still unknown. They may act directly on the ARAS (296, 328, 331), but there is no electrical synchronization (recorded with macroelectrode) to corroborate this hypothesis in decorticate animals. Actually, no synchronization (spindles or slow waves) appears during behavioral sleep at the midbrain tegmentum level in chronic decorticate preparations (240). In posterior mesencephalic or chronic pontine preparations (which have no behavioral sleep comparable to slow sleep) a wide exploration of the brain stem in chronic conditions has not yet allowed us to record reticular synchronizing phenomena during periods preceding PS (240, 242).
Consequently, if tonic synchronizing structures do exist in the lower brain stem, there is no evidence their synchronizing activity acts directly on the ARAS; in fact, it may act on more rostral structures, diencephalic or cortical. There is no evidence that ARAS is only "inhibited from behind," for other facts are in favor of deactivation "from in front."
Descending hypothesis. PHYSIOLOGICAL EVIDENCE. On the one hand, the thalamus appears to be necessary for the occurrence of spindles, since its destruction by coagulation (293), section (109), or aspiration (337) abolishes cortical spindles during onset of sleep while cortical slow waves persist.
On the other hand, after complete removal of the neocortex (240, 251, 418), there appears an immediate permanent disappearance, lasting several months, of the synchronized or slow activity at the subcortical structures (thalamus or reticular formation) level, even after pentobarbitone injection (251, 404). How ever, the recruiting response (RR) obtained by medial thalamic stimulation may persist at the RF level after decortication (396, 400, 401), but it is likely that the RR is induced by mechanisms different from those provoking the sleep spindles, since it is not accompanied by pyramidal responses typical of spindles (72). On the contrary, if even a small part of the gyrus orbItal.is and coronalis anterior is left undamaged, reticular slow waves may persist during sleep (240) in subtotally decorticate chronic cats (240). This fact suggests that the basal part of the cortex is essential for the subcortical synchronization of slow sleep. This hypothesis has found support in the recent experiments of Velasco and Lindsley (424). In acute conditions, ablation of the entire dorsal convexity and of the medial and cingulate regions of the cortex failed to interfere with the spindle bursts whereas ablations confined to the orbItal. cortex alone abolished completely these potentials in both cortex and thalamus. Therefore the orbItal. cortex appears to be the only region of the neocortex to play a crucial role in the regulation of the thalamo-cortical synchronizing function.
Other experiments are also in favor of a descending cortical synchronizing influence on the brain stem but they do not permit us to localize a specific cortical region.
The functional depression of the cortex (by local application of KCl), which involves the "spreading depression of Lao" suppressing the cortical EEG, abolishes the reticular spindles and slow waves (54, 432, 434).
After a total mesodiencephalic section of the brain stem, cortical and thalamic spindles persist in front of the section, but none can be recorded caudal to the site of the section (240, 251).
Unilateral, only cortical, interventions (removing of the dura mater) involve cortical homolateral synchronizing phenomena (419).
These data permit us to infer that the synchronized slow activity observed at the brain-stem level during slow sleep necessitates the presence of the cortex, and most probably the orbItal. cortex, and therefore that there are synchronizing but not obligatory sleep-inducing structures at a rostral level.
MODE OF ACTION. The activating influence of the cortex on the ARAS has been repeatedly demonstrated in acute (70) as well as in chronic conditions (403) and has been confirmed by the demonstration of postsynaptic excitatory potentials by cortical stimulation at the level of the reticulospinal neurons (299). On the other hand, the mechanisms of inhibitory action of the cortex on the ARAS are still almost unknown. To date, it has not been possible to elicit postsynaptic inhibitory potential (by intracellular recording at the ARAS level in acute experiments) after cortical stimulation (299, 300). However, there is some evidence suggesting that the cortex may have an inhibitory descending influence, either on the ARAS or on the facilitatory descending reticular system.
On one hand, gamma activity is depressed during cortical spindles (76, 214); on the other, cortical stimulation can inhibit, in a lasting and cumulative way, the unitary activity at thalamic level (282), but no direct proof of this action has been yet found at the hypothalamic or mesencephalic tegmentum level. According to Krnjevic et al. (283), this cortical inhibition would be the expression of an eventual noncholinergic chemical agent.
DESCENDING PATHWAYS. The descending pathways responsible for synchronizing phenomena at the brain-stem level still remain largely unknown. Nevertheless, we know the part of the pyramidal tract that transmits the spindles of cortical origin (8, 437). Pyramidal projections have also been described at the pontobulbar RF level (190, 297). The limbic midbrain circuit (339), and particularly the medial forebrain bundle, may constitute a descending pathway, receiving inhibitory influences from the neo- and paleocortex. The impingement point of the limbic midbrain circuit with the ARAS would then be located at the level of the limbic midbrain area described by Nauta (339); it must be admitted, however, that lesions at this level do not suppress slow sleep (95, 206).
SUBCORTICAL SYNCHRONIZATION AND SLEEP. In the neodecorticate animal, the presence of behavioral sleep phases (240), preceding PS, was established, apart from any synchronizing phenomenon at the thalamic or mesencephalic tegmentum level. Therefore we must conclude that ARAS synchronization is not a necessary condition for the occurrence of a sleep behavior. Nevertheless, the existence of high-voltage spikes at the rhinencephalon during this state (240) suggests the eventual role of a paleocorticofugal descending influence normally added to the neocorticofugal synchronizing influence, thus accounting for sleep in de corticate animals. The inhibitory (but not synchronizing) action of the rhinen cephalon at the ARAS level has been described in acute experiments (6). How ever, there is as yet no proof that the high-voltage spikes observed either at the level of the rhinencephalon or in its efferent pathways (240) exert an inhibitory activity acting on the hypothalamo-reticular arousal system.
There are thus many concordant facts that favor the existence of behavioral and EEG sleep-inducing structures in the lower brain stem, and it is possible that these structures belong to a group of serotonergic neurons located in the raphe nuclei. On the other hand, the orbItal. cortex, which seems to play a de terminant role in the appearance of the EEG pattern of slow sleep (cortical and subcortical spindles and slow waves), is not, however, necessary for the appearance of behavioral sleep.
A possible explanation of these apparently contradictory facts would be that the sleep-inducing structures of the lower brain stem may act directly on the ARAS without generating synchronization.
The orbItal. cortex would then secondarily assume its necessary synchronizing influence, either through a direct action from the lower brain-stem structures or indirectly through the decrease of the activating effect of the ARAS.
Whatever may be the interpretation of the contradictory facts reported, supporting either of the hypotheses trying to delimit the structures responsible for behavioral drowsiness and for synchronization, other experimental results suggest that slow sleep may be actively induced by both central and peripheral stimulations. These are based on the results of stimulation and that is why they will be treated separately, for they produce no definite conclusions in favor of either the ascending or the descending hypothesis.
Results of electrical and chemical stimulations. CENTRAL TRIGGERING OF SLOW SLEEP. This field was opened by Hess' classical experiments (202, 203). In chronic experiments in cats (145) low-frequency (from 3 to 15 cycles/sec) stimulation of numerous structures in the brain may induce spindles or cortical slow waves with or without sleep behavior (as a matter of fact, we know that the presence of recruiting cortical waves by thalamic stimulation may be observed during behavioral arousal) . That is why, successively, the following have been described or suggested as "hypnogenic" structures some cortical areas frontal, somesthetic cortex; anterior and posterior suprasylvian gyrus (82), visual and motor cortex (347, 355); head of the caudate nucleus (77, 78); the internal capsule (186); the preoptic region (102, 103, 199, 412, 413, 443); dorsal (349) or ventral hippocampus (264, 349); the amygdala (280, 281); the anterior (150, 264, 349) or posterior (348) hypo thalamus; mamillary bodies (349); the thalamus massa intermedia (13, 203); diffuse thalamic system (14, 222, 317, 318); the interpeduncularis nucleus (348, 349); the mesencephalic (157, 191, 259, 257) or pontine (90, 382, 383) reticular formation; the cerebellum (448); and the medulla region of the solitary nucleus (295, 296). So almost the whole encephalon has hypnogenic capacities. That is why the localizing value of hypnogenic electric stimulations is little justified the interpretation of the results yielded by this method indeed meets numerous difficulties. The stimulation may not act on neuronal cell bodies at all but rather on their afferent or efferent axons. On the other hand, one stimulation may excite both synchronizing and activating elements, though most stimulations are of low frequency (that of spindles). Moreover, it is difficult to know whether sleep is induced by the stimulations or has been spontaneously produced, for most in vestigators agree that the occurrence of sleep is favored by stimulating a "relaxed" animal and that it is extremely difficult to induce sleep during a state of intense alertness, except with long repeated stimulations (375). In fact, a careful survey of the numerous experimental results in which EEG and/or behavioral sleep has been induced by cerebral stimulation is quickly discouraging and we have been unwilling to undertake such a difficult synthetic work, so imprecise and varied are the criteria used to define sleep. We refer the interested reader (who trusts the effect of hypnogenic cerebral stimulations) to Parmeggianl's recent paper (349), which is one of the most fully documented. Our personal experience, during which we have stimulated hundreds of chronic cats over a period of several years, has not convinced us that a cat, asleep after any stimulation (other than the painful ones), would not have gone to sleep spontaneously. Actually, if a hypnogenic intracerebral stimulation is effective, it is because the numerous bodily needs responsible for wakefulness are fulfilled heat, food, comfort, etc. It is possible that some intracerebral stimulation may induce behavioral or/and EEG sleep in a hungry, frightened female cat during estrus, exposed to a cold environment, but in such an unusual case, it would certainly not be a "physiological sleep." This methodological problem again explains why research about sleep is so protracted and yet also so fascinating.
Finally, it must be pointed out that sleep induced by central stimulation has only been observed in animals with an intact cortex. Stimulation of the thalamus in neodecorticate animals does not induce sleep (240).
The solution expected from chemical stimulations in situ is not yet forthcoming. As there are serious criticisms as to the specificity of the action of the drugs (366), such techniques have no greater localizing value than have electric stimulations. The areas in which chemical stimulations are claimed to induce slow sleep, either EEG or electrical and behavioral, become more and more numerous, and in a few years they may cover all the places where electric stimulations induce sleep. Though adrenaline injected in situ at the brain-stem level has a clear arousing action, the proof of the arousing action of adrenaline or noradrenaline is, however, not yet definitely established (see 35); the injection of adrenaline into the carotid has no activating effect (94). On the other hand, in young birds and kittens whose blood-brain barrier is permeable, adrenaline injection induces behavioral sleep (268, 307). Likewise, a state "resembling sleep" may be obtained by injecting adrenaline at the level of the ventricles (158). Acetylcholine or serotonin (445) injected in microcrystals appears to have a real hypnogenic effect. Injected at the level of the caudate nucleus (445), the preoptic region (197, 198), the medial thalamus (445), the pontine reticular formation (109), and the limbic midbrain circuit (199), they may produce slow sleep states, though sometimes these states seem more like coma because it is so difficult to wake the animals (197).
PERIPHERAL REPLEX INDUCTION OF SLOW SLEEP. "Slow sleep" may be induced not only by cerebral stimulation but also by the stimulation of numerous afferent systems e.g. auditory stimulations (378), repetition of insignificant tones during habituation (238, 389), or the repetition of tones that have acquired an inhibitory signal value (in Pavlovian terminology) (327, 388), intermittent photic stimula tions (21), stimulation of the cutaneous (Group II) or muscular nerves (360, 361, 378). The synchronizing cortical action of pressure applied to the skin (285) has also been described. Vegetative afferent influences may also induce drowsiness vagus (118, 354) or laryngeal stimulations (369), probably acting via vagal afferents, may induce EEG, ocular, and behavioral signs of sleep. Finally, the profound sleep-like state observed by Koch (278), caused by stimulation of the depressor nerves, may also induce an EEG pattern of sleep in the encephale isole, where no change of blood pressure is produced (60). All these findings show that slow sleep may be actively induced but do not give any knowledge about these active mechanisms. On the one hand, the role of these stimulations does not appear to be exclusive, since the encephale isole animal, with buffer nerves and vagi transected (disconnected from most vegetative and muscular afferents), still exhibits "slow sleep." On the other hand, the central locus of action of external hypnogenic stimulations is still unknown. Bulbar structures on which baroceptive impulses are directly impinging do not appear to coincide with the solitary tract "synchronizing" area (295), and the synchronizing tone of the lower brain stem does not seem to be triggered by barosensitive afferents (108). The fact that EEG synchronizing afferents of Group II chiefly project at the gigantocellularis nucleus level (in decerebrate animals) (362) does not allow us to assume the intervention of synchronizing caudal brain-stem structures, for, very likely, more rostral projections might be observed in the intact animal. Finally, we must mention it is relatively easy to synchronize and induce sleep in preparations with an intact cortex, including midpontine pretrigeminal preparations (21, 304) (by flashes of light), whose level of arousal is, however, much increased. This action supports the intervention of a descending rostral synchronizing mechanism. We have still no evidence that sleep can be induced by external stimuli in decorticate or in anterior mesencephalic preparations.
III. State of sleep characterized by fast cortical activity paradoxical sleep
Since early times, hunters have noticed that during sleep their dogs showed sudden motor episodes (tail and lip movements, barking), and Lucretius [Denatura rerum. IV, 984-I004, quoted by Moruzzi (328)] long ago attributed these episodes to oneiric activity. Fontana (161), as far back as 1765, called "sonno profondo" this sleep with "convulsions." But 20 years were necessary, from the first electrical description, to integrate this state of sleep into neurophysiology. When EEG began to be applied to chronic experiments, Derbyshire et al. (130) reported fast cortical activity periods "as in the alert waking state," when sleep was less quiet "as judging by twitching of vibrissae." This observation was confirmed by Rheinberger and Jasper (371). At the same time, Klaue (274) described, at length, the two states of sleep in chronic cats and published the first EEG recording of the state of "Tiefen Schlaff" with fast cortical activity and low voltage. This work was unfortunately forgotten. In spite of short descriptions of cortical desynchronization [Hess et al. (204)] or of hippocampal theta thythm [Rimbaud et al. (373)] during sleep, we must recognize that until 1958 only the state of "slow sleep" was known and studied in the cat. It is to the credit of Dement and Kleitman (122, 126, 127), studying man and the cat, to have characterized definitely the periodical recurrence of activated sleep with rapid eye movements. This state of sleep was then interpreted as an intermediate phase beween slow sleep and arousal. Soon after the demonstration that this sleep was, in fact, "deeper" than slow sleep and could indeed be identified by a specific subcortical electrical activity (pontine spikes) and specific postural criteria (total atony) (252) led to the demonstration of its rhombencephalic origin and to the hypothesis that it was a different state of sleep (252, 254). Since then numerous observations were made about EEG and be havioral correlations (42, 217, 246, 277) (see bibliography in 240) of the state of sleep with fast cortical activity, whose paradoxical aspects gave it the name, among many others, of paradoxical sleep (254) (see footnote 2).
A. Behavioral Aspects
Somatic phenomena
Contrary to slow sleep, in which the behavioral criteria are imprecise, the onset and the end of paradoxical sleep (PS) may be fixed and assessed within a few seconds from mere behavioral criteria not only in intact cats but also in decorticate or chronic pontine preparations. Behavioral phenomena may be classified into two types tonic and phasic.
Atonia. The complete abolition of muscular tone of antigravity muscles and, above all, of neck muscles, is the most remarkable manifestation of the inhibition of muscular tone, typical of PS (254). Preceding or following, by a few seconds, the cortical desynchronization of PS, an electromyogram (EMG) silence accompanies the sudden fall of an animal's head. The end of PS is shown usually by a sudden recovery of a considerable EMG activity, whether the animal wakes or "falls" again into slow sleep. This very typical neck muscle atonia may be found alike in gamma (decerebrate animal preparations) (240) or in alpha (decerebrate animal preparations with removal of the anterior lobe of the cerebellum) type of spasticity (206). It is also observed after section of posterior roots from C1 to C7 (233,242)
Spinal refllexes. Accompanying this atonia, or even preceding it by a few seconds, a decrease or even a disappearance of heteronymous monosynaptic and plurisynaptic reflexes may be observed (171, 181-183, 284, 358, 420), whereas usually these do not vary much during slow sleep. The homonymous monosynaptic reflexes are not tonically inhibited during PS but only during the bursts of eye movements (358). Last, the posttetanic facilitation of monosynaptic reflex (obtained by stimulating the posterior roots) is abolished during PS but persists during slow sleep (25, 183).
Clonic movements. Among phasic phenomena typical of PS, eye movements are predominant and consequently are considered separately. They occur with cortical activation and their pattern is different from that of the eye movements of the waking state. However, they are not isolated and other phasic movements accompany (in a strange unpredictable manner) PS development sudden move ments of the ears, the vibrissae, the fingers (flexion) (see 172), the tail, and some times genuine clonic jerks of the back muscles. These phasic phenomena are especially developed in the kitten after birth (86, 236, 422, 423) and increase in a striking way after long PS deprivations (237, 429). The animal then seems to be animated with convulsions ["comincio a tremare tutto quasi fosse in qualche convulsione" (161)] .
Vegetative phenomena
These are constant in the cat and also express tonic and phasic changes in the vegetative sphere. A fall in blood pressure appears at PS onset (90, 170,261); it may be interrupted by short hypertensive phases during bursts of ocular movements. It is accompanied by a great irregularity in heart rate (bradycardia or tachycardia depending on the animal) (240), and it may also be observed when PS is induced by brain-stem stimulation (90). The falls in blood pressure are much larger after bilateral sinoaortic deafferentation; they fall to such low pres sure during PS that episodes of transient cerebral ischemia (EEG flattening and seizures) sometimes occur (189). But an artificial fall in blood pressure (caused by vagal stimulation) does not induce PS (90). The mechanisms of action of this fall in blood pressure are still unknown. The fall is not dependent on the muscular hypotonia since a blood pressure rise is often the first sign of the end of a PS episode, before the return of EMG activity (90, 170). Cerebral blood flow has been studied by methods using the shifts of cerebral temperature or of cerebral impedance (50, 260, 261). The most striking phenomenon is the large increase in blood flow that occurs during the generalized fall in blood pressure (260, 261).
Several hypotheses have attempted to explain this blood flow increase action of a cerebral vasodilatation or an increase in cerebral metabolism expressing itself by an augmentation of CO2, which is a well-known cerebral vasodilatator (260). An increased cerebral temperature at the onset of the fast cortical EEG of PS has also been reported (266, 367). Respiratory variations are also noticeable; most of the time, they consist of an irregularity and increase in the rhythm, whereas it is usual to observe an apnea at the end of PS (240).
The galvanic skin reflex (GSR), either spontaneous or induced by stimulating the peroneal nerve, has also been studied during PS (420). Most of the time (70 % of the cases), there is a notable decrease of both spontaneous and induced GSR during PS in comparison with slow sleep. Sometimes spontaneous GSR may appear in bursts during PS; interestingly enough, these bursts are not concomitant with the rapid eye movements.
Arousal threshold
If we trust the following criteria, PS appears as a deeper state of sleep than slow sleep; behavioral arousal threshold by reticular stimulation is much increased (up to 300%) in comparison with slow sleep (42, 90, 217, 254). This arousal threshold increase may also be observed in decorticate animals (240), which eliminates the hypothesis of a possible inhibitory corticoreticular feedback (221). The arousal threshold by auditory stimulation increases too, either slightly or sometimes greatly (96, 150, 240), and above all some stimulations, unable to induce behavioral arousal, involve the reappearance of slow sleep (217,240).
Other behavioral (muscular atonia) and vegetative (blood pressure fall) criteria also support the idea of a deeper level of sleep during PS. That is why this state of sleep is sometimes called "deep sleep" (90), opposed to "light sleep" (slow sleep). As a matter of fact, the concept of depth or "heaviness" of sleep is ambiguous and essentially depends on the criteria used. The possibility that some learning could occur during sleep (with conditioning methods) has been extensively studied (79, 80). Thus it appears that some classical conditioning is still possible during slow sleep whereas it is almost absent during PS. On the other hand, judging by the phasic movements of the eyes or of the legs, PS rather appears as a "restless, less quiet" sleep (130). It is not certain either that the increase in "depth" of PS in comparison with slow sleep is constant in all animal species (55), or even that the arousal threshold remains constant during PS in the cat (in correlation with blood pressure shifts) (90). Moreover, PS and slow sleep increasingly appear to be two qualitatively different states, and hence it is perhaps rather illusive to compare them from the quantitative ambiguous point of view o f "depth" or "lightness."
III. State of sleep characterized by fast cortical activity paradoxical sleep
B. Electrophysiological Aspects
Paradoxical sleep (see Fig. 1) in an unrestrained normal adult cat takes place after a variable period of slow sleep. It appears then periodically during slow sleep, and mean duration is 6 min (but periods from 15 to 20 min can be frequently recorded). Its percentage in comparison with the duration of be havioral sleep is from 20 to 25% (about 15% for a period of 24 hr) (120) (see Table I). As in the behavioral aspects, it is also possible to recognize in the electrical cerebral activity during PS two major components tonic (fast cortical ac tivity and regular theta hippocampal activity) and phasic (monophasic ponto geniculo-occipItal. spike activity associated with rapid eye movements).
TABLE 1. Stability of sleep patterns of the cat calculated with method of continuous polygraphic recordings
| Awake | Slow sleep | Paradodixal sleep | |
| % total time | 31.5+-7 (28+-4.2) | 52.5+-7 (56.5+-5.3) | 16+-2 (15.5+-2.2) |
| % total sleep time | - | 77+-4 (72.4+-4.2) | 23+-4 (27.5+-4.2) |
| Mean duration | - | (107.2+-12.9) | 5.8+-1.3 (5.8+-1.3) |
Modified from refs. 120 and 413 (in parentheses). Means and SD were obtained in g animals (120) and in 8 animals (413).
Tonic activity
Tonic activity is characterized by a neocortical diencephalic and mesence phalic low-voltage fast activity (20-30 cycles/sec), which is similar to the cortical desynchronization that usually accompanies intense arousal or attention states (analysis of cortical EEG frequencies does not always allow a discrimination between both types of activity) (79, 342, 420). However, some electrical local cortical and subcortical activity enables us to discriminate the electric cerebral activity of PS from that of behavioral arousal. The appearance of a continuous theta rhythm at the level of the ventral and dorsal hippocampus is most characteristic (85, 188, 240, 288, 289, 342, 350, 351). It is steadier and faster (5-7 cycles/ sec) than that observed during intense wakefulness (4-4.5 cycles/sec) at the level of the dorsal hippocampus (but it can be recorded also in the ventral hippocampus where theta rhythm occurs only occasionally during intense arousal). The hippocampal activity seems to have an autonomous reactivity. Indeed, the sensory stimulations may involve a fast hippocampal activity without any shift of cortical activity. Yet sometimes a short burst of cortical spindles may accompany the archeocortical activation. Theta rhythm has been also recorded at the level of the pulvinar (12), the periacqueductal grey matter (240), and the anterior part of the pons at the level of the limbic midbrain area (242). The olfactory bulb activity shifts also in a characteristic way, for the sinusoid rhythm from so to 60 cycles/sec, observed during arousal, disappears during PS (154, 263, 270).
Unit activity and steady potential. The PS cortico-subcortical fast activity is accompanied by an important increase of unit activity compared with slow sleep and even with arousal.
At the level of the cerebral cortex, the frequency of the discharges increases (139, 140). Evarts (142) has studied the discharges of the pyramidal tract neurons. Their total statistical activity remains the same during arousal and PS (just as the integrated background of pyramidal tract activity increases at the same level as wakefulness during PS) (20). Yet the bursts of increasing unit activity occurring during the phasic movements of PS are different from those of arousal. Evarts suggests this difference in pattern indicates a disinhibition of the inhibitory cortical interneurons even more important than that occurring during slow sleep, which would involve a "disorganization" of the cortical unit activity during PS (139), At the level of the mesencephalic tegmentum, the increased unit activity is important too (often twice as much as during a relaxed wakefulness). According to Huttenlocher (223, 224), occlusion but not inhibition would account for the decrease in unit responses to clicks in the RF during PS. The results concerning the steady cortical potentials are not in agreement. In the rat, Caspers (97) observes a positive shift at the onset of PS (therefore in the same direction as slow sleep). On the other hand, Dement (125), Rossi et al. (385), and Wurtz (442) in the cat and Kawamura and Sawyer (265) in the rabbit have noticed a negative shift (therefore in the same direction as in cortical arousal). These last findings agree with the shifts of cortical and subcortical impedance (50), which are similar to those observed in arousal. Thus PS seems to be the appearance of a state qualitatively different from that of slow sleep.
Phasic activity
The very close relationship between the electrical phasic activity and the visual system makes it necessary to study these in the same section.
Rapid eye movements. Rapid eye movements (REM) appear at the onset of the cortical activation. From 60 to 70 movements a minute, their rapidity and frequency, their "pattern," enables us to distinguish them from the movements of observation during waking (229-231). Either isolated or in groups of small bursts of less than 5 movements (as can be noticed also during observation), they are mainly characterized by the presence of bursts including more than 5 movements (up to 50 closely following one another). The ratio between the total number of the movements and those within the bursts is constant in every animal (50 %) during PS (231). The myosis is maximal most of the time (240), whereas the nictitating membranes are relaxed. Yet, at times, a sudden mydriasis with retraction of the nictitating membranes may accompany the volleys of ocular movements (46, 47, 212). This phasic pupillary dilatation remains even after ablation of the superior cervical ganglia and therefore must be ascribed to an inhibition of the tonic activity of the Edinger-Westphal nucleus (46, 47). The analysis of the structures responsible for the appearance of eye movements isolated and in bursts has given the following results (231) the pontine cat (superior colliculus destroyed) has only isolated lateral and external movements (depending on the VIth nerve); in the mesencephalic cat (superior colliculus intact) more important bursts of ocular movements persist. On the contrary, the coagulation of a zone located at the level of the superior colliculus and of the mesencephalic tegmentun in the intact animal suppresses the bursts. These bursts, in turn, are much increased in the decorticate animal. But the role of the cortex is not unequivocal, because the removal of the visual cortex strikingly reduces the isolated eye movements and the bursts, whereas a frontal decortication or a frontal leucotomy produces a very marked increase in these bursts.
It has recently been shown that the destruction of the medial and descending vestibular nuclei suppressed also the burst of REM, whereas isolated eye movements were still present during PS. Those nuclei in which unit activity is increased during PS (53) apparently control most of the phasic phenomena of PS (322).
Thus, the REM of PS belong to mechanisms different from those of wake fulness since they still exist in preparations utterly incapable of ocular movements during arousal (such as decorticate or pontine cats) just as they are present during PS in newborn kittens that are still blind (422, 423). The results support the hypothesis that these ocular movements are triggered at the level of the vestibular nuclei and that a growing "complexity" is involved at the level of the superior colliculi and of the mesencephalic tegmentum, whereas processes of "cortical integration" (facilitating visual cortex and inhibitory frontal cortex) would impinge back on the latter zone (231).
Phasic electrical ponto-geniculo-occipItal. activity. The difficulties and delays of a wide and systematic exploration of the cortical and subcortical structures in chronic experiments explain why it took several years before a link was obvious between the "spontaneous" phasic potentials observed during PS. First described at the level of the pontine RF (254), 200- to 300-,uv monophasic spikes 100 msec in duration, often occurring in groups of 5-6 (hence their look of pseudo-spindles), could be observed later at the level of the lateral geniculate nucleus (74, 196, 315) (Fig. 2) and at the level of the occipItal. cortex (334), the superior colliculus and the nucleus of III (74, 312), the pulvinar and the parietal cortex afterward (207). Pontine and geniculate phasic spikes are the first electric signs heralding the appearance of a PS episode. They may appear 1-2 min before the cortical activation and the disappearance of the neck EMG (74, 312) and sometimes occur erratically during slow sleep. They may occur sporadically during 5 % of slow sleep time (429), and usually during PS they have a frequency of 60-70 min.
The latency between the monophasic pontine potentials and the geniculate potentials is very short (5 msec) (52). Geniculate evoked responses having the same pattern as spontaneous spikes can be evoked (gating effect) through stimulation of the pontine RF during PS (then they have a 25- to 35-msec latency) (52). But it is impossible to evoke geniculate responses through stimulation of the pons during arousal or slow sleep. The selective triggering of the ponto-geniculo-occipItal. (PGO) activity by reserpine (119) has recently allowed the study of the organization of the PGO activity in acute experiments using Flaxedil (227, 248) (see below).
The relationship between this phasic activity and the REM is not simple neither darkness nor coagulation of the retina (47), nor even complete removal of the eyes and of the extraocular muscles (312), suppresses these PGO spikes (at least during the 2 or 3 days after the operation). Therefore, this activity cannot be considered a possible feedback of a retinal "on and off" effect of extrinsic muscular origin. Moreover, this phasic activity appears 30 or go sec before the ocular movements at the onset of PS. There sometimes may be ocular movements without any recorded activity of spikes, but in most cases there is a close time relationship between the phasic PGO spikes and the muscular activity of the extrinsic muscles (314); the latter mainly occurs in phasic bursts, whereas there is a tonic component during arousal.
These data are in favor of there being an ascending pontine extraretinal projection at the level of the lateral geniculate and the occipItal. cortex. Similar extraretinal input to the lateral geniculate body has been noticed after stimulation of the mesencephalic reticular formation or the labyrinth (258). Besides, lesions of the occipItal. cortex can show signs of degeneration at the level of the pontine RF [raphe nucleus and nucleus reticularis pontis caudalis (136)], thus favoring the existence of a system of projection between the pons and the visual cortex.
Whatever the complex organization of the extraretinal input to the lateral geniculate body might be, it is very likely that the optic tract terminals are in volved in the genesis of geniculate monophasic spikes. Indeed these spikes disappear some 6 days after enucleation of both orbits (232, 333) or retinal photocoagulation (51) though REM's and pontine spikes persist (333). The time course of wave disappearance corresponds to that of optic nerve degeneration. The hypothesis that PGO spikes could be induced by the release of monoamines at monoaminergic terminals impinging on pontine neurons or on optic tract terminals in the lateral geniculate is summarized below (119, 248).
Unit activity. The unit activity of the occipItal. cortex strikingly increases during PS in comparison with slow sleep. This increase of the discharges reaches its peak during eye movements, even while the animal is in the dark. It can be compared then to the discharges recorded during visual observation (140). These unit discharges during PS may be the unitary translation of the monophasic spikes recorded with macroelectrodes, although the latter were not recorded. On the other hand, at the level of the optic nerve, there is an important decrease of unit activity during slow sleep and PS (41) that contrasts with the increase of activity at the level of the lateral geniculate nucleus and of the mesencephalic reticular formation during PS (224). However, a transitory arrest of the spon taneous activity of l2 % of geniculate units belonging to on-off and off-on cells) was observed during monophasic spikes by Bizzi (51).
Evoked responses
Though the interpretation of the various components (early or late) of the evoked cortical responses is still a matter of discussion, the purpose of many experiments has been to analyze their shifts during the various states of sleep. The results are different according to the specific systems that have been stimulated, the nature of the stimuli, the cortical areas studied, and the methods used to estimate the results. The use of automatic techniques, which is rapidly spreading, to record averages by means of a "memory computer" has greatly simplified the task of experimenters. But in working out an average amplitude (founded on series of 50-100 responses) some significant short-lasting shifts may be concealed (for example, during some phasic events of PS). We shall briefly survey the responses evoked by physiological stimuli at the level of the receptors (shocks, clicks, flashes) and those evoked by electric stimulation of the afferent specific or non specific pathways or relays during the two states of sleep.
Responses evoked by stimulation of receptors.
AUDITORY SYSTEM. The decrease and variability of the primary (fast) and secondary (late) responses during slow sleep compared to arousal have long been known (68). There is no noticeable variation in amplitude at the level of the ascending relays. On the other hand, the responses decrease in amplitude in the mesencephalic RF compared with relaxed wakefulness (440)
During PS the amplitude of the cortical responses shows a decrease (240, 440) comparable to the one observed in intense arousal. The decrease in amplitude is also noticeable at the level of the mesencephalic tegmentum and has been ascribed to an occlusion phenomenon (223). At the level of the cochlear nucleus the responses may disappear, especially during REM bursts (240). This amplitude reduction is not noticed after section of the middle-ear muscles and must therefore be ascribed to a peripheral phenomenon (34, 13l ) and not to the intervention of a possible central control of afferent activity.
SOMESTHETIC SYSTEM. Whereas the cortical evoked responses to the radial nerve stimulation increase during slow sleep (the associative responses, mainly), in PS the amplitude of the responses increases during the early phase in comparison with slow sleep, but decreases in the late phase (16, 342).
VISUAL SYSTEM. A decrease in cortical evoked responses to flashes of light has been observed during PS, and a total depression of evoked responses was described whenever spontaneous geniculo-occipItal. spikes occurred (51, 334).
Responses evoked by stimulation of specific afferent pathways. The most permanent fact is the increase in the cortical response to the stimulation of an afferent pathway or of a specific thalamic nucleus [optic nerve, lateral geniculate nucleus, medial lemniscus, nucleus ventralis postero lateralis (VPL), medial geniculate nucleus] in comparison with slow sleep. This phenomenon is similar to the facilitation of the cortical responses by reticular stimulation (69, 134). According to some authors, the increase of the responses is similar to those observed during intense arousal (106, 110, 342, 346). For others, on the contrary, this facilitation is even more dramatic (357, 385). All the findings reveal an increase in the first phase of the responses compared with slow sleep, whereas contradictory results are obtained concerning the late phase of the visual response (110, 145). Some experiments suggest that the facilitation of the responses may take place at the thalamic level (155, 156), whereas others favor facilitation at the cortical level (26, 110, 342, 385). The most interesting results concern the interaction between the spontaneous phasic geniculo-occipItal. activity and orthodromic and antidromic responses of the optic tract. A reduction in optic tract orthodromic response and an increase in optic tract antidromic response to lateral geniculate stimulation (whereas flash-evoked responses were depressed in the lateral geniculate and in the cortex, though not in the optic tract) are consistent with the occurrence of presynaptic inhibition at optic tract terminals during the monophasic spikes that occur during REM (51,226). On the other hand, the increase in the postsynaptic component of the evoked lateral geniculate responses during the spontaneous spikes favors the existence of facilitation at the postsynaptic side (226). This mechanism may explain why the decrease in flash-evoked cortical responses is observed during PS at the same time that a facilitation of the geniculate-induced cortical responses is obtained.
Responses evoked by stimulation of nonspecific systems. The recruiting response elicited by a low frequency stimulation of the diffuse thalamic system (increasing a great deal during slow sleep) is very much reduced in PS (342,385,444). This reduction is achieve to alevel comparable to (385) or even lower than that during arousal (420,446).
The data of theses experiments have been discussed in detail elsewhere (110,385). They enable us to draw a rather surprising conclusion : that the cortical excitabitiliy would be much enhanced during PS to alevel that may even be superior to that of intense arousal.
III. State of sleep characterized by fast cortical activity paradoxical sleep
C. Structures and Mechanisms responsible for Paradoxical Sleep
Triggering structures
The existence of tonic and phasic behavioral signs (disappearance of EMG activity on the neck, rapid eye movements) and of subcortical electric signs (monophasic pontine spikes), both of which are very specific for PS, has enabled us to outline withe relative precision the structures necessary and sufficient for the periodical triggering of this state of sleep (239,240).
The complete removal of the cerebellum (240) or of the anterior lobe (206) does not hinder the appearance of PS, which occurs in the normal way with the same behavioral signs seen in the intact animal. Thus the cerebellum, whose role in the disappearance of the postural tonus might be justly involved, is not necessary for the appearance of PS.
In the neodecorticate animal, PS is characterized by the appearance of the steady theta activity at the level of the ventral hippocampus, by monophasic pontine spikes, and by peripheral signs similar to those in the intact animal. The decrease of the muscular tone is total, and the bursts of eye movements are still present [though they have a pattern different from the normal animal (231)], as well as the other phasic muscular phenomena (clonic movements). The PS periodicity and duration are similar to those of the intact animal. Thus the neocortex does not play any part either in the triggering of PS, in the initial development of the hippocampal theta rythm, or in the most charactristic peripheral manifestation (240).
The removal of all neural structrures (including hypothalamus and hypophysis) rostral to the pons (242) does not prevent the periodical appearance of PS in the chronic pontine animal : it occurs with the regularity of a "biological clock", its mean duration is the same as in the intact animal (6 min.), and its circadian percentage is 10 % (slightly less than that in the intact animal). Paradoxical sleep is characterized by the sudden disappearance of muscular tone with atotal decrease of the EMG of the neck muscles, and by lateral ocular movements (which depend on the activity of the IVth nerve), an acceleration of the heart and respiratory rythms, and the presence of monophasic pontine spikes whose pattern and regional distribution are tha same as in the normal animal during this state. The structures responsible for the triggering of PS therefore must be located behind a prepontine transection.
On the contrary, in two animals, after a prebulbar transection from the posterior two-thirds of nucleus reticularis pontis caudalis to the rostral border of the trezoid body (240), it was impossible to observe a periodical disappearance of the muscular tone, and the EMG activity remained constant at the necck level during survival time (7 days). Thus, the bulbar inhibitory reticular formation (303) makes necessary a prebulbar mechanism, since by itself, it can not trigger the recurent inhibition of the tonus in these last preparations. The ocular and electric cortical aspects in these preparations are the same as in the MPP preparation [ a clear increase in the duration of cortical desynchronization, accompanied by ocular movements of observation (240)]. Moreover, there are periods of fast cerebral activity with spontaneous and short-lasting eye movements. These periods might be the expression of the rostral activity of PS, but this has not yet been proved. Yet the hippocampal activity in the dog is the same as in PS after midpontine transection (406).
The experiments of Rossi et al. also supports the hypothesis of the localization of the trigerring structures of the fast activity of PS at the level of the caudal part of nucleus reticularis pontis oralis (RPO) and of the rostral part of nucleus reticularis pontis caudalis (RPC), since a lateral hemisection located in front of these sructures suppresses or delays the homolaeral cortical desynchronization during PS, whereas an hemisection at a more caudal level does not involve an asymetry of the cortical desynchronization during this state (92,382,384).
The overall results of these experiments reveal that the structures sufficient fot the periodical appearance of the major behavioral and EEG signs of PS are located at the level of the pons.
Another series of experiments shows that some pontine structures are equally necessary.
The destruction of the nucleus centralis superior de Betcherew and of the medial part of nuclei RPO and RPC has no significant effect on either states of sleep. But the coagulation of mediolateral part of the caudal part of nucleus RPO and the rostral part of nucleus RPC suppresses PS in chronic cats, whereas they are not usually changes in slow sleep or arousal (239,240,242). Recent experiments are in favor of different pontine structures being responsible for both tonic and phasic components of PS.
Tonic inhibition of muscular tonus. The bilateral destruction of a limited area situated in the rostral part of the mediolateral pontine tegmentum suppresses the occurrence of muscular atonia during PS (247) (whereas the phasic PGO activity still occurs). This area includes the nucleus locus coeruleus and a zone immediately medial and ventral to it. The destruction of a zone situated more caudally (anterior group of vestibular nuclei) or more laterally (nucleus parabrachialis-brachium conjunctivum) had no effect on the total atony of PS. Behavioral disturbances may occur in animals in which the dorsal part of the mediolateral pontine tegmentum is destroyed (247). After a period of slow sleep, a sudden increase in lateral geniculate spikes occurs, whereupon the cats may suddenly stand up and exhibit behavioral fear or rage. During these episodes, which occur periodically, there is an augmentation of muscular activity of the neck but the pupils remain fissurated, the nictitating membranes are relaxed, and the animal does not react to visual stimuli. The clear-cut dissociation between the ocular aspect of "deep sleep" and the behavior of rage has been compared to a "hallucinatory-like state." How the dorsal part of the mediolateral pontine tegmentum controls the supraspinal structures responsible for the tonic inhibition of muscular tonus is still obscure. It must be pointed out, however, that this region is very rich in noradrenergic neurons [group A6 of Dahlstrom and Fuxe (114)], which are mainly concentrated in the locus coeruleus and immediately ventral to it. This region is also particularly rich in mono-amino-oxidase ( 193) . These histochemical features favor a possible role of monoaminergic neurons in the control of postural atonia during PS but further experimental evidence is needed to support this hypothesis.
Phasic aspects of PS. The medial and descending vestibular nuclei are re sponsible for the burst of rapid eye movements, for the clonic jerks of the peripheral muscles, and for some phasic irregularity of the vegetative system (subite mydriasis or subite variation of blood pressure) during PS. Indeed, after bilateral destruction of these vestibular nuclei, there are only a few isolated eye movements without clonic jerks whereas there is still total atony of the neck muscles (322, 359).
The pontine structures that control the phasic PG0 activity during PS are less known. However, it has been recently shown that reserpine could induce electively, in the cat, PGO spikes similar to those of PS (119, 248). This activity can still be recorded in acute experiments under Flaxedil. In this condition neither any retropontine transection of the brain stem nor even total destruction of the vestibular nuclei can suppress the PGO activity induced by reserpine, whereas a total midpontine transection or the bilateral coagulation of the lateral pontine tegmentum (rostral to the vestibular nuclei and ventral to the locus coeruleus) will totally suppress the geniculo-occipItal. spikes (248). Thus the lateral pontine tegmentum is apparently necessary for the triggering of ascending extraretinal input to the lateral geniculate body.
The rhombencephalic localization of the structures responsible for the triggering of PS has made it possible to propose the name of "rhombencephalic phase of sleep" for this state of sleep (254). The mediolateral zones of the rhomben cephalon thus appear as triggering zones in relationship to some ascending and descending pathways, responsible for the electric cortical tonic and phasic phenomena and the behavioral tonic and phasic events specific to PS.
Ascending and descending structural organization in relationship with the pons
Ascending structures. The ascending structures responsible for the "cortical activation" in PS have not yet been delimited. We must discard the suggestion (241) that they follow the "limbic midbrain circuit," since coagulations at this level do not prevent cortical activation during PS (95, 206). These structures seem to be diffuse and nonspecific since neither the destruction of the rostral part of the reticular formation of the pons and of the specific pathways located in front of the rhombencephalic triggering zone nor the coagulation of the mesencephalic tegmentum suppresses this activation (91, 206). The destruction of the septum, on the contrary, suppresses the hippocampal theta rhythm in PS as well as during arousal (240, 288, 350, 351).
Cortical "activation" in PS. A total section of the brain stem at the level of the mesodiencephalic border (destroying the posterior diencephalon) suppresses the cortical activation during PS, whereas the pontine electrical signs and the peripheral signs of PS are still observed (206, 240, 252).
These results lead us to the conclusion that the cortical activation during PS cannot be attributed to a humoral action exerting a direct influence on the cortex (240). On the other hand, the number of experimental results obtained is still too small to enable us to know whether the cortical activation of PS is due to a neuronal, neurohumoral, or a humoral activation of the ARAS, or to other ex trareticular diencephalic structures. Moreover, the nature of the cortical activation must be considered from the point of view of two aspects
I) does the cortical ac tivation of PS reveal an increase or a decrease of the cortical "excitability," and
2) are its mechanisms different from cortical "arousal"?
I) Though it is difficult to agree on the meaning of cortical "excitability," a substantial body of data is in favor of there being an increase in cortical "excitability" during PS (in comparison with slow sleep state)
a) increase in the unit discharges of neurons at the visual and pyramidal cortex (128, 140, 142);
b) increase in the background activity of the pyramidal tract (20);
c) a high level of responses of the cortical units to flashes (141) and the presence of monophasic visual cortical spikes (334);
d) increase in the geniculate evoked cortical responses and decrease of the "recovery cycle" at this level (40, 110, 385);
e) increase in the cortical excitability by direct stimulation of the cortex, as tested by the appearance of peripheral responses (213) [on the other hand, responses of the flexor muscles to pyramidal tract stimulation decrease in PS (306)] ;
f) increase in the discharges elicited by local application of strychnine in the cortex to a rate similar to arousal, whereas these discharges decrease during slow sleep (341). It has not yet been established whether this increase in excitability is of a tonic nature (and would remain constant as long as the fast cortical activity would last) or of a phasic nature (in close linkage with motor phasic phenomena of PS).
2) Some results are in favor of a dissociation of the ascending "activating" mechanisms of PS and of arousal.
a) Some lesions located at the level of the diencephalon (subthalamic area, lateral hypothalamus, thalamectomy) involve a dissociation between the activations of wakefulness and those of PS, which implies the existence of different ascending pathways or mechanisms (218, 240, 337).
b) The unitary cortical activities of arousal and PS are different, at least in their patterns. It has been suggested that a difference of excitability of inhibitory interneurons could be the source of the unorganized discharges of pyramidal tract neurons (142).
c) The pattern of the evoked cortical responses during arousal is often different during PS (see above).
d) The presence of spontaneous spikes at the visual cortex (334) level (which does not exist during arousal) implies that some mechanisms different from those of arousal are working or that there is a new activity superimposed on arousal activity.
e) Last, the topography and the frequency of the hippocampal theta rhythm are different from those observed during wakefulness (240, 420) and the olfactory bulb activity is different from that of arousal (154, 263, 270).
Thus, all these data enable us to infer that the mechanisms of the neo- and paleocortical activation of PS are different from those of arousal.
Supraspinal influences on motoneurons. If the dorsal part of the mediolateral pon tine tegmentum appears to be necessary for the periodical atony of PS, other supraspinal structures exist in the lower brain stem that are under its control and can act on the spinal motoneurons. These structures have been the subject of a recent review (358); the hypothesis of an inhibition of the descending tonic activity exerted by the facilltating RF or by the nuclcus of Deiters is unlikely, since partial spinal transection that interrupts facilitatory influences (182) dose not prevent the inhibition of the spinal reflexes. On the other hand, there is no decrease in unit activity at the level of the nucleus of Deiters or of the superior vestibular nucleus in PS (53). That is why the hypothesis of an active intervention of the inhibitory bulbar activity seems the most likely (240). This hypothesis is supported by the results of Giaquinto et al. (181, 182), which, in interrupting the descending inhibitory reticulospinal pathways at the level of the ventrolateral quadrants of the spinal cord, suppress the characteristic inhibition of the monosynaptic spinal reflexes during PS. The distal jerks of the limbs, which generally occur at the acme of inhibition of the spinal reflexes (172) during the bursts of rapid eye movements, are accompanied by an increase in the activity at the level of the pyramidal tract (306). Yet they do not depend en tirely on the pyramidal bursts or on the gamma loop, for they still exist in the ani mal without its neocortex (240), with sensorimotor areas removed, with both pyramids destroyed, or after section of the posterior roots (306). These jerks are, on the contrary, strikingly reduced by section of the dorsolateral funiculi of the spinal cord (306).
To sum up, it seems that the inhibition of the muscular tonus, of the homo and heteronymous monosynaptic reflexes, of the polysynaptic spinal reflexes, and of the pyramidal motor responses can be attributed to an inhibitory supraspinal influence having its source, probably, at the level of the inhibitory bulbar reticular formation. This inhibition would exert an influence (pre- or postysnaptically) on the spinal reflex arcs. A detailed analysis of the descending influences on the spinal cord has been carried out with elegant techniques in chronic conditions. Two tests have been used by Pompeiano et al. (173-175) to evaluate the excitability of a population of spinal motoneurons during sleep
1) the recurlent discharge (RD) of the alpha-motoneurons, which can be recorded electromyographically in the deaflerented limbs;
2) the re sponse of a muscular nerve to direct stimulation of the motoneuronal pool after deafferentation of the limbs.
Both the RD of the alpha-motoneurons as well as the response of a muscular nerve to direct stimulations of the motoneuron pool are depressed throughout PS. This tonic reduction of the response of the motoneurons to antidromic or direct stimulation is due to hyperpolarization of the alpha-motoneurons produced by descending inhibitory volleys, and this postsynaptic inhibition is also responsible for the tonic depression of the homonymous monosynaptic reflexes tested in the same population of motoneurons during PS.
The phasic inhibition of the monosynaptic reflexes that occurs during the bursts of eye movements, however, had no counterpart in enhanced depression of the response of the motoneurons to antidromic or direct stimulation.
It has also been shown that the phasic depression of spinal reflexes during the bursts of eye movement of PS is the result of presynaptic inhibition of the group 1a afferent pathway. During experiments in which the excitability of group 1a muscle afferents was tested in chronic cats following Wall's method, a phasic increase of the antidromic Ia volley appeared particularly when the bursts of ocular movements were rather intense. There was, however, no significant change in the amplitude of the group Ia antidromic volley during PS compared with slow sleep, when the bursts of eye movements were absent (323).
Thus it is likely that the increase in excitability observed during PS is due to supraspinal influences exerting a synaptic depolarizing action on terminals of the group 1a primary afferents. These influences are phasic in nature and appear to be related in time with the large bursts of eye movements. This phasic presynaptic inhibition may account for the striking depression of the homorlynlous monosynaptic reflex that occurs during the bursts of eye movements (358).
The complex organization of the neural structures involved in SP may be sum marized as follows. There are some structures at the level of the pontine reticular formation that appear necessary for the triggering of both descending and ascend ing tonic and phasic phenomena of PS.
The mediolateral pontine reticular formation is involved in the control of the bulbar inhibitory reticular formation, which acts tonically on the motoneurons through the ventrolateral funiculi of the spinal cord. The medial and descending vestibular nuclei are responsible for the burst of rapid eye movements and for most phasic vegetative and muscular events of PS. Phasic influences are transmitted through the dorsolateral funiculi of the spinal cord.
The fast cortical activity (and regular theta hippocampal rhythm) and the geniculo-occipItal. spikes are also dependent on the mediolateral part of the pontine tegmentum. The ascending pathways responsible for the tonic cortical activation appear to be diffuse at the level of the mesencephalon, whereas the hippocampal theta rhythm during PS is controlled by a pathway ascending through the septum.
A very discrete pathway, independent from the above ascending structures, situated in the dorsal part of the brain stem is responsible for the pontine ascending extra retinal input to the lateral geniculate body and occipItal. cortex.
Triggering mechanisms of paradoxical sleep
Central triggering. It is possible to trigger or "hasten" the appearance of PS im mediately or after a latency of a few seconds, in the intact animal, by a ihigh-fre quency stimulation (200-300 cycles/sec) of the pontine (240) mesencephalic RF (90, 152) or by a low-frequency stimulation of the hypothalamus (150, 264) and the hippocampus (152, 351). Induced PS resembles spontaneous PS in all points, including the decrease in blood pressure (90). This phenomenon appears only if the stimulation is made during slow sleep and several minutes after the end of a preceding PS episode, which implies the existence of a refractory phase (240). This triggering can also be realized in the mesencephalic or pontine animal (240) by stimulation of the pontine RF. Since PS is a spontaneous recurrent pherlomenon, relatively regular, some authors question whether this "central induction" is possible and assign it to chance in the rat and the rabbit (379, 380).
Chemical intracerebral stimulation (acetylcholine) at the level of the pontine RF (109, 177) and of the limbic midbrain circuit (199) can trigger PS too, but the necessary precedence of a phase of slow sleep does not enable us to learn whether this phenomenon would not have occurred spontaneously.
Reflex induction of PS. A direct triggering of PS cannot be obtairied during wakefulness in the normal cat by stimulation of extracerebral afferences. Yet, the stimulation of the Group II fibers of the cutaneous afferences during slow sleep in the intact cat can involve the phasic inhibition of the muscular tone of the neck [as well as a decrease in the monosynaptic contralateral reflex and polysynaptic flexion reflex (179, l80)], and in some cases this stimulation could "hasten" the appearance of PS (358, 360). On the contrary, in the chronic pontine cat, with a section behind the mesencephalic tegmentum, nociceptive stimulations (pinching of the ear) or proprioceptive stimulations (passive flexion of the legs or of the head) can trigger PS as a reflex. This phenomenon cannot be obtained in the anterior mesencephalic cat in which the same stimulations involve hypertonus (242). On the one hand, we must admit that the peripheral stimulations do not set off the inhibitory bulbar reticular formation directly, but rather pontine structures near (or the same as) the zone necessary to the triggering of PS, which is located at the dorsomediolateral part of the pontine RF. On the other hand, the integrity of the mesencephalic tegmentum suppresses the appearance of this phenomenon by a possible triggering of the descending facilitatory influences. Last, in the pontine animal, stimulations are efficient only if they occur l0-l5 min after the end of a period of PS (spontaneous or induced), otherwise they are ineffective or can involve a phasic or tonic reflex neck atony (lasting 2 or 3 min). The latter phenomenon similar to cataplexy resembles the "sudden posture collapse" described~ by Bard and Macht in the chronic pontine cat (27), but it is not accompanied by the appearance of monophasic pontine spikes, eye movements, nor vegetative shifts during genuine reflex periods of PS.
The possibility of triggering PS through reflex pathways thus raises the problem of a physiological triggering by extracerebral afferents or by a muscular factor (294). In fact, neither the section of the posterior roots of Cl to C7, nor the total section of the spinal cord in Dl, nor the section of both vagi and of the buffer nerves, nor the removal of the cervical sympathetic nerves prevents the appearance of PS in the intact animal (233, 242), and therefore we can reject the hypothesis of an exclusive triggering of PS by any afferent nervous pathways of extracerebral origin.
Humoral influences. The peculiar conditions required for the appearance of PS in the rabbit have made it difficult to recognize it. Its very existence has even been refuted by some authors (55). In fact, it is now admitted (17, 149, l50, 263) that PS corresponds to what has been formerly described in the female rabbit in terms of "hyperarousal" (394) or "afterreaction" of EEG (395).
Paradoxical sleep appears spontaneously too in the male rabbit but it requires long habituation of the animal to the environment (150) for it to become apparent. In the female rabbit, PS shows close relationships with hormonal changes. Copulation is usually followed (after a latency of l0-l5 min) by a period of PS, whereas PS is followed by a complex olfactory-buccal-anal-genItal.-sexual behavior (149 151, 153, 263, 394). The injection of placentary or pituitary gonadotrophins, of LH and mainly LTH, of ADH, oxytocin, and epinephrine encourages the appearance of PS (150, 394, 395). Moreover, an injection in situ of LH at the level of the nucleus reticularis pontis caudalis can cause the appearance of PS (l50). But the injection of testosterone in castrated females or of synthetic progestative drugs (inhibiting ovulation) would suppress, for I or 2 days, spontaneous PS or PS in duced by central stimulation (394). As a matter of fact, PS can be induced in the rabbit by a low-frequency stimulation of various structures having close relation ships with the hypothalamus (hypothalamus, olfactory bulb, septum, hippocampus, amygdala) (l50, 151, 264, 430). These stimulations are able to exert an influence by triggering hypothalamic neurohormones or pituitary hormones (395), and this raised the problem that a suprapontine command (or modulation) of PS triggering may exist. Yet, the hypothalamo-pituitary complex does not seem necessary to the appearance of PS, at least not in the cat. The complete removal of the hypothalamus and hypophysis (in the pontine cat) is still compatible with the appearance of PS during the first 5 days after the operation (242). Its percentage steadily decreases down to the death of the animals on the 6th or 7th day. On the contrary, an injection of ACTH and ADH restores the normal periodicity of PS. Thus the pituitary hormones (which completely disappear from the blood within a few hours after ablation of the hypothalamus and hypophysis) do not seem to be necessary for the appearance of PS. They thus intervene only in an indirect way to allow survival of the animals by restoring an ionic blood equilibrium that would otherwise be disturbed.
The influence of the blood osmolarity on the periodicity of PS is very important in the pontine animal (with a hypothalamic island disconnected from the pons by a barrier in acrylic resin) (242). The blood hyperosmolarity, through injection of a hypertonic saline or water deprivation, elevates the percentage of PS by about 100-200 %. The hypoosmolarity induced by a water surcharge ( 10 % of the animal's weight) suppresses the appearance of PS for 6-12 hr. The interactions between the blood osmolarity and the brain (415) and the Na and K contents of the neuroglia (whose role in the cerebral water-ion pool is known) have led to the suggestion of a possible intervention of the glial cells in PS triggering (242).
Selective deprivation of paradoxical sleep
The first attempt to suppress PS electively was made in man by Dement (123) by awakening the subjects immediately after the onset of PS. A progressive in crease in PS "attempts" was observed and an augmentation of PS was found dur ing the nights after deprivation. The same results have been obtained in animals.
In the pontine cat, the suppression of PS by an electric shock makes it reappear after shorter and shorter intervals, so that after a few hours it becomes almost im possible to awake the animal, which immediately collapses into the state of PS after the shock (242). Thus, there is a state of "a need for PS" and it seems to be the expression of a quite active mechanism situated in the lower brain stem. The same phenomenon has been obtained in the intact cat with the same technique (343).
In the intact cat, the selective deprivation of PS is also achieved by placing the animal under conditions in which it cannot completely relax its muscular tone (isolated on a small stand in a bath) (237, 429). Under these conditions, behavioral and EEG slow sleep can persist in a normal way (50 % of the day) without PS appearing. Some vegetative electrical and behavioral changes have been noticed during PS deprivation. There is a permanent increase in the heart rate (237, 429), a facilitation of the recovery cycle of click-evoked responses at the cortical level (132), and a diminution of the seizure threshold for electroshock (105). Disturbances of sexual behavior (hypersexuality) have also been noticed in male cats or rats (132, 429). These results suggest that the excitability of the nervous system may be increased when PS is electively suppressed. During recovery sleep (after suppression for several days) an important and durable increase of PS percentage is observed (up to 60 % of total sleep). This increase may persist for several days and is proportional to the duration of deprivation (it lasts for a duration equal to half the deprivation time). It proceeds mainly from an increase of the frequency of PS and not from the increase of its mean duration. At the onset of the recoveries, PS may occur immediately after wakefulness, without any intermediary phase of slow sleep. The phenomenon evokes cataplectic states, thus showing that PS can sometimes occur without a preceding slow sleep state. The elective suppression of PS has also been obtained in the rabbit by subjecting it to continuous intensive noises. Return to silence is followed by a "rebound" recovery of PS (269). The results of elective PS deprivation contrast with the effect of total sleep deprivation. In such case the recovery sleep is different. There is first an increase of SWS, which is only secondarily followed by an increase of PS (429). Similar results have been obtained in man (439).
These experiments demonstrate that the essential mechanisms expressing the "need" for PS exist in the lower brain stem. The long-lasting recovery process after PS deprivation evokes the eventual accumulation of a neurohumoral or metabolic by-product during deprivation and meets Pieron's theory of hypnotoxins (356). The increase of this neurohumoral agent appears to be responsible for a state of increased excitability of the nervous system. The recovery after PS deprivation necessitates a long-lasting increase of PS, suggesting that the elimina tion of the specific (and unknown) neurohumoral agent during PS is effectuated through some autoregulating mechanism whose frequency (but not duration) is increased.
Pharmacological influences
The literature concerning the pharmacology of sleep is so important that a whole review could be devoted to it (see the most recent bibliography in ref. 187). For this reason we shall limit our review mostly to the pharmacological influences on PS.
The active mechanism responsible for PS proves to be relatively resistant to certain factors. A decrease in rectal temperature down to 30 C during artificial hypothermia does not prevent the recurrent appearance of PS in the pontine animal (240). PentobarbItal. (25-30 mg/kg) does not suppress the recurrent appear ance of PS in the neodecorticate animal (290). It is easy to detect in these preparations thanks to the appearance of pontine spikes, grouped in periods of 6 min, that clearly stand out against the ever-fast and low-amplitude basic activity of the brain stem. In the intact animal, the cortical and subcortical spindles during narcosis under Nembutal (30-35 mg/kg) have long delayed the conspicuousness of PS; yet it still appears (234). It is evinced by typical pontine and geniculate spikes grouped in regular periods of 6 min, without cortical activation or REM at the onset, whereas these signs gradually reappear at the end of the narcosis. It is interesting to notice that some increase in pontine and geniculate spikes is also observed, after selective PS deprivation, during recovery under Nembutal narcosis (234).
Among the numerous drugs used for understanding the mechanisms of sleep this review is limited to three groups whose action is especially interesting.
Short-chain fatty acids. The artificial induction of PS by gamma-butyrolactone (GBL) or gamma-hydroxybutyrate of sodium (GNA) was first recognized by Jouvet et al. (242, 245). In the intact animal this drug (50-60 mg/kg) causes the appearance of a sleep similar to slow sleep in its EEG characteristics, which is always followed almost immediately by PS. Higher doses (above l00 mg/kg) provoke a state of narcosis with an electrical activity different from that of slow sleep (spikes and slow waves) and PS does not appear. But in the decorticate or pontine animal, the injection of GBL even in 100-mg/kg doses induces the appearance of PS, which always occurs in periods of 6 min mean duration. But it reappears more often and its percentage rises from 200 to 300 % after the injection. GBL is unable to induce PS m animals with lesions of the pons (which do not show spontaneous PS) (245). The intimate mechanisms of action of GBL or of GNA are still unknown (it is still under discussion whether they are normal components of the brain or not) (38, 48, 49, 160, 184, 185, 287). However, it has been shown recently that other short-chain fatty acids can also induce both states of sleep in normal cats or, electively, PS in mesencephalic cats (310). Sodium butyrate, isobutyrate, isovalerate, or caproate and a-hydroxyisobutyrate were effective, whereas sodium propionate, acetoacetate, and ,alpha-hydroxybutyrate were not effective in producing PS.
Cholinergic and anticholinergic drugs. In normal conditions, atropine sulfate (1-2 mg/kg) suppresses PS for several hours both in normal or pontine cats, whereas eserine increases the duration of PS in pontine cats (240). However, after selective PS deprivation, when the "need for PS" is enhanced, atropine does not suppress the immediate appearance of behavioral slow wave sleep followed by the usual increase of PGO activity during recovery sleep. Thus the preliminary phenomena of PS (phasic PGO activity) are not suppressed by anticholinergic drugs. On the contrary, the final steps of PS (fast EEG activity and total disappearance of EMG) are more sensitive to atropine, and they reoccur only after several hours (428) . Thus, if it is possible that some cholinergic mechanisms could play a role in the tonic phenomena of PS (low-voltage fast cortical activity and postural atonia), these mechanisms are not involved either in the production of slow wave sleep or in the triggering of the phasic EEG phenomena of PS.
Drugs acting on brain monoamine level. Whereas the facilitatory effect of short chain fatty acids or the suppressor effect of atropine on both states of sleep is short, the effect of drugs acting on brain monoamines is usually long (several days), and only the method of continuous polygraphic recordings associated with selective deprivation of PS has made the study of their action possible.
INCREASE IN BRAIN MONOAMINES.
I) Injection of precursors. The injection of s-hy droxytryptophan (5-HTP) (30-50 mg/kg), which is the precursor of serotonin (5-HT), provokes in normal cats an increase of slow wave sleep for 5-6 hr, whereas PS is totally suppressed during this period. The immediate and elective suppressor effect of 5-HTP on PS is also observed if 5-HTP is injected at the beginning of recovery sleep after PS deprivation. But the administration of 3,4-dihydroxyphenyl alanine (DOPA) (30-50 mg/kg), which is a precursor of the catecholamines, induces an increase in the waking state with an almost total disappearance of SWS and PS for 6 hr.
2) Blockage of metabolism of monoamine. Another way to increase the monoamine level is to block the mono-amino-oxidases (MAO) with an inhibitor. Potent MAO inhibitors (Nialamide, Pargyline, Ipronazide) increase mostly the 5-HT brain level in the cat. These drugs increase SWS and electively suppress PS (256). The suppressor effect of Nialamide is quite dramatic; after a single injection PS is totally suppressed for 3-4 days and SWS is increased, and a normal level of PS is only reached after 1 week. The same suppressor effect is also observed after PS deprivation. The very potent and elective suppressor effect of MAO inhibitors on PS has led to the hypothesis that the transition from SWS to PS may necessitate the inter vention of MAO (243).
RELEASE OF BRAIN MONOAMINES ACTION OF RESERPINE. The decrease in brain 5-HT and catecholamines after reserpine is a well-established fact (118, 309) even if the intimate mechanism of action of this drug is still unknown. In the cat, reserpine (at a unique dose of 0.5 mg/kg) induces a very peculiar alteration of the sleep states, which can be summarized as follows
1) it suppresses SWS for 12-14 hr;
2) it totally suppresses PS for 22-24 hr, and the control level of PS is reached only after 5-6 days of recovery;
3) it electively triggers a permanent PGO activity that lasts for 50-60 hr.
About 40-60 min after a single injection of reserpine, after a brief period of agitation, the cat shows the well-known reserpine syndrome with a fissurated myosis, but may still react to loud noise or painful stimuli. At the same time there is a fast cortical activity with some discrete low-voltage spindling at 11 cycles/sec lasting continuously for 10-12 hr. Periods of SWS with high-voltage spindles reap pear after 12 hr and a normal level of SWS is reached on the following day.
At the onset of the behavioral changes induced by reserpine (40-60 min after the injection) some clusters of PGO spikes appear. They are first isolated and may be separated by intervals of some minutes. During the 2nd hr after injection they become permanent, with a frequency of 40-60 min. They are usually unique, but frequently bursts of 6-8 monophasic spikes, entirely similar to those of PS, appear. This PGO activity is similar in all respects to the PGO activity of PS. It can also be recorded at the same level of the pons, mesencephalon, lateral geniculate, and occipItal. cortex. It is accompanied by very discrete lateral eye movements, small twitches of the vibrissae, ears, and even "fingers." But there is no decrease in the EMG of the neck, and the behavioral aspect of the cat is quite different from that of sleep. This PGO activity is not suppressed by external stimuli and may increase during the period of agitation. It lasts permanently for 40 60 hr with the same frequency and disappears slowly.
When the first brief episode of behavioral and polygraphic PS appears (after 22-24 hr), this activity becomes more irregular and clusters of monophasic spikes increase. The decrease in PS lasts for 4-5 days and the normal level of 15 % PS is usually reached on the 6th day. Thus reserpine has the unique property of electively and permanently inducing the PGO activity without affecting the other tonic components of PS. Since reserpine is known to depress the level of both 5-HT and catecholamines in the brain, the precursors of both amines were injected after this drug in order to increase them selectively.
1) Reserpine + 5-HTP. 5-HTP (30-50 mg/kg) injected 2-3 hr after reserpine immediately (within 1or 2 min) and totally suppresses the PGO activity induced by reserpine in 4-6 hr; and induces EEG and behavioral signs of SWSÑsuppres sion of fast cortical activity and appearance of high-voltage spindles (periods of SWS reappear for 4-6 hr, after which the usual fast cortical activity and PGO activity of reserpine reappear).
2) Reserpine + DOPA. DOPA (30-50 mg/kg) injected 2-3 hr after reserpine increases (after 10-15 min) the frequency of PGO activity by about 30-50 % for 4-6 hr; and induces (after a latency of 50-60 min) brief periods of SWS that are often followed by behavioral and polygraphic PS (with total extinction of the neck activity and clusters of rapid eye movements). Thus during the 5-6 hr that follow DOPA injection, two or three periods of PS may appear (and an almost normal level of SWS and PS is reached during this short period). When the effect of DOPA has disappeared (after 5-6 hr), the PGO activity returns to the base-line level (40-60/min), and the reserpine syndrome reappears.
Thus the association of reserpine with precursors of both monoamines shows the importance of serotonergic mechanisms in the occurrence of SWS, whereas the induction of the tonic phenomena of PS when DOPA is injected after reserpine suggests that a catecholaminergic mechanism may be involved in the total atony of PS. A more detailed analysis of drugs acting on monoamines in relationship with sleep mechanisms has been published recently (243, 244).
The actual state of the neuropharmacological approach on sleep mechanisms could be summarized as follows.
There is no unique and continuous hypnogenic mechanism presiding over the periodical succession of the states of sleep since it is possible, by altering the brain monoamine level, to increase SWS and to suppress PS. If there were a single sleep mechanism for both states of sleep, PS, which is considered deep sleep, would also have been increased (or at least would not have been suppressed).
Increase of the brain 5-HT level led to an increase in SWS (and a parallel decrease of PS).
Some metabolic step requiring MAO appears to determine the transition from SWS to PS.
The release of monoamines (whether 5-HT or catecholamines or both) at monoaminergic terminals induced by reserpine triggers electively the appearance of the phasic EEG components of PS.
The total atony of PS appears to be dependent on cholinergic mechanisms (since it is suppressed by atropine) and to necessitate also a catecholaminergic mechanism since DOPA is able to induce normal PS after reserpine.
Thus, the neuropharmacological approach to a sleep mechanism, still at its beginning, has revealed at least two different mechanisms in the process governing the transition from SWS to PS.
The first mechanism (the appearance of PGO activity, which always heralds the occurrence of PS) is totally suppressed by an MAO inhibitor (but not by atropine) whereas the second mechanism (which triggers total atony of PS) is dependent on both cholinergic and catecholaminergic mechanisms. The mode of action of short-chain fatty acids able to act rapidly on these different mechanisms is still totally unknown.
III. State of sleep characterized by fast cortical activity paradoxical sleep
D. A Synthesis of Paradoxical Sleep Mechanisms
Even though it seems perhaps premature, we may try to distinguish between the facts and the hypotheses concerning the two major problems about PS
1) which are the intrinsic mechanisms, and
2) which are the triggering mechanisms?
1) Numerous data suggest that PS is accompanied by an increase in neuronal activity an increase in unitary cortical and reticular activity (140, 224), background level of pyramidal activity (20), and cortical excitability (estimated from evoked responses). However, it still remains to be proved whether this increase of activity is due to the mere intervention of the ARAS (at a level identical with or superior to that of intense wakefulness) or if it is a different mechanism (as might be thought from the disorganization of cortical unit activity). Then it should be assumed that PS is the manifestation of an intense disinhibitory state (superior to that of slow sleep) of the cortical and reticular neurons (but that during waking an inhibitory control would be exerted only on the former) (142).
However, PS does not seem to be the expression of the same neuronal processes (even at cortical level) underlying slow sleep (142). The data of cerebral impedance (50), cerebral temperature (266, 367), cerebral blood flow (261), and cortical steady potential (442) are, on the contrary, in favor of the appearance of a new state that is in accordance with the neuropharmacological data. The problem of ascending structures, ascending from the pons and responsible for fast cortical activity and for occipItal. and geniculate monophasic spikes, has not yet been totally solved. The persistence of cortical activation after important lesions of the specific or nonspecific formations of the brain stem makes a neurohumoral mechanism very likely.
Whatever the efferent results of this tonic and phasic increase in neuronal activity may be, a tonically active, very powerful mechanism triggers from the pons the inhibitory reticular formation that blocks, at a pre- or postsynaptic level, the discharges of spinal motoneurons (except phasic discharges and ocular movements). This intervention of the inhibitory RF might account for some of the contradictory aspects concerning the arousal threshold. To induce behavioral arousal, the electrical stimulation of the ARAS must indeed run the blockade exerted by the inhibitory RF at the motoneuron level, when the ARAS may itself be in its maximum state of excitability. Then PS might be compared to a hyperwakefulness state in a preparation under curare (but blocking of the efferents would be at the spinal and not the neuromuscular level).
2) On the one hand, PS represents the expression of an active process; as a matter of fact, it may be triggered by high-frequency stimulations of the brain stem and abolished by local coagulation at the pontine level. Moreover, this whole mechanism is resistant to hypothermia and the PGO phasic activity is somewhat resistant to Nembutal narcosis.
The triggering cause of PS does not appear to be necessarily situated at the pontine level after pontine RF coagulations preventing PS, periodical behavioral disturbances (hallucinatory-like state), perhaps expressing the reaction of the nervous system to the cause (humoral or neurohumoral) that would normally trigger PS, may be observed. Pontine triggering structures appear as effectors with ascending or descending action. The existence of a refractory phase, during which it is impossible to trigger PS by stimulation, compels us to admit that there is a triggering threshold in the intrinsic cause of PS, although the intrinsic periodicity of PS (and its limited duration even after long deprivations) supports the theory of a self-regulating process.
Though numerous factors, hormonal (in the rabbit), humoral (hyperosmolarity), or pharmacological (short-chain fatty acids), may facilitate PS, the triggering factor is still unknown, nor are the triggering mechanisms of the periodical rhythm of PS in pontine animals. It cannot be ascribed to a hypothalamopituitary factor nor to the action of a nervous peripheral mechanism. Finally, the presence of numerous noradrenergic neurons in the dorsal part of the mediolateral pontine tegmentum associated with the dramatic effect of reserpine suggests that PS could be the expression of some mechanism involving cerebral monoamines. We do not know its subjective aspect in animals, of course, but numerous data strongly sug gest that oneiric activity occurs during PS in man.
III. State of sleep characterized by fast cortical activity paradoxical sleep
E. Relationship With Oneiric Activity in Man
The assimilation of the paradoxical phase of sleep in the cat with the oneiric activity phase (REM phase) in man has raised many arguments. Today, if we compare the paradoxical phase of sleep in the cat and the REM phase in normal man (24, 121, 126, 127, 168, 233, 249, 257, 377) or in patients suffering from various cerebral lesions (255, 353), there is no doubt about the similarity of the phenomena (241, 249, 408) (Table 2) and that there is "a need" (first elicited in man) "for oneiric activity" appearing during selective deprivation experiments (123, 125). The demonstration of the relationship between oneiric activity and REM sleep in man allows us to speculate that mechanisms and structures, progressively elicited in the cat during PS, will enable us to understand eventually the causes and functions of the oneiric state. Moreover, subjective data given by subjects awakened when dreaming, immediately after REM, seem to reveal a close relationship between the direction of ocular movements and the oneiric scenery ( 124, 377) . It then appears possible that postsynaptic geniculate and occipItal. events originating in the pons (described in the cat) may be responsible for oneiric imagery in man. Numerous questions are still to be settled before explaining the relationship of REM to this imagery. As a matter of fact, as in the cat, rapid eye movements in man during sleep have patterns and speeds different from those of visual observation, but resembling those of ocular movements during attempts to remember events (228); on the other hand, PS ocular movements persist in subjects suffering from a decortication syndrome, unable to have ocular movements during wakefulness (249, 255). Moreover, REM occur during sleep in newborn infants (376) and in blind-born adults (43, 345), who cannot have visual imagery during dreaming. If phasic electrical and oculomotor phenomena both appear to be triggered from the pontine area, the precise determination of the temporal relationship existing between ponto-occipItal. spikes and REM is essential in order to understand the integrating mechanisms responsible for oneiric imagery.
TABLE 2. Similarities between paradoxical sleep in the cat and REM sleep in humans
| EEG | Cat (adult) | Man (Adult) |
| Cortical activity | Fast, low voltage (similar to arousal) | Scalp recording: low-voltage, 6-8 cycles/sec in occipItal. areas |
| Evoked responses by visual or auditory stimuli | Decreased | Average response decreased |
| Physiological behavior | Cat (adult) | Man (adult) |
| EMG Activity | EMG Activity of the neck totally decreased | Subhyoidian muscle activity totally decreased |
| Monosynaptic reflexes | Decreased or abolished | Decreased or abolished (H-reflexes) |
| Ocular movements | Present | Present |
| Pupillary diameter | Myosis | |
| Heart rate | Mostly decreased, irregularity | Mostly increased, irregularity |
| Blood pressure | Decreased | Increased but different method |
| Respiration | Irregularity | Irregularity |
| GSR | Decreased | Decreased |
| Cat (adult) | Man (adult) | |
| Functionnal aspects | - | - |
| Arousal threshold | - | - |
| Auditory stimulation | Increased | Superior or equal to stage III equal to stage IV |
| Periodicity | 20-28 % of total sleep time, always follows slow sleep | 20-25 % of total sleep time, always follows slow sleep |
| Result of selective deprivation | Increased during recovery | Increased during recovery |
| Structural aspects | Persist after decortication Suppressed by pontine lesions | Persist in case of decortication Suppressed by brain-stem lesion involving the pons |
| Ontogenesis | Present immediately after birth, may directly follow waking | Present immediately after birth, may directly follow waking |
| Subjective experience | Unknown | Dreaming |
From Dement, W. C., and M. Jouvet. General discussion. In Aspects anatomo-fonctionnels de la physiologie du sommeil, edited by M. Jouvet. Paris Centre Natl. Rech. Sci., 1965.
IV. Phylogenesis of the states of sleep
The phylogenetic study of sleep allows us to dissociate (250), during the course of evolution, the appearance of slow sleep from that of PS. In reptiles (tortoise, for instance) only the state of slow sleep was found and PS could not be observed (201). In birds (chicks, hens, pigeons), a "slowsleep"state is quite typical and electrically resembles that of mammals (275, 276, 421) though there are no spindles and few slow waves, and PS is extremely short (10 sec). However, PS is quite distinct it is accompanied by a short acceleration of the electrical activity of the hyperstriatum, by an important reduction but not by total disappearance of the neck muscle tone, by bursts of phasic ocular movements, and by bradycardia. Until now, all mammals observed [rat (75, 96, 313, 417), mouse (433), rabbit (17, 148, 263, 435), opossum (407), cat and dog (55, 405), sheep (235), goat (391, 392), macacus (436), and chimpanzee (5, 372)] present the two states of sleep just studied. In certain species a few special features are known, e.g. in the rabbit PS is hormonally dependent and a long habituation is necessary in order to observe it. On the other hand, in newborn ruminants, the two states of sleep may be easily observed; PS tends to decrease considerably in adult animals (235, 392) and this may explain why PS has not been described before (37).
V. Ontogenesis of the States of Sleep
If phylogenesis allows us to dissociate the appearance of the two states of sleep during evolution, such a dissociation may also be observed during ontogenesis in the cat. In kittens and newborn rats (84, 86, 236, 422, 423), PS may be observed immediately after birth, when slow sleep is almost nonexistent. Paradoxical sleep has a peculiar aspect phasic phenomena prevail on tonic phenomena, which is why it is called "sleep with jerks" (422) or "sommeil agite" (86). It occupies about 50 % of the day and 80-90 % of behavioral sleep and very often occurs immediately after waking, since there is no phase of slow sleep between. Progressively, during cortical maturation, a slow sleep state appears and increases while PS decreases, so that in the adult, slow sleep constitutes 70 % and PS only 20-25 % of behavioral sleep. The occurrence of fast cortical activity (423) and of hippocampal theta rhythm (86) during PS precedes, by a few days, the waking tonic cortical desynchronization, which was interpreted in favor of a dissociation of the mechanism respectively responsible for waking and PS fast cortical activities.
VI. Relationship between slow sleep and paradoxical sleep or duality of sleep mechanisms
Reciprocal interrelations between slow sleep and PS are obvious PS only occurs in normal subjects (cat or man) after a slow sleep phase lasting a variable period, and that is why the theory of the unicity of sleep was set forth. According to this theory (197), an ascending and descending hypnogenic process would first affect the ARAS at the limbic midbrain area level (and so would account for the slow activity of slow sleep) and then would progressively invade, upward, the thalamic synchronizing structures (which would account for the disappearance of the recruiting response) and would thus provoke a fast cortical activity through inhibition of the thalamic synchronizing structures. However, the absence of any relationship between the duration of the previous slow sleep period and that of PS, and the persistence of a fast cortical activity after destruction of the thalamus, defeats any attempt to define the hypothetical mechanisms responsible for the invading of "inhibition" in the brain stem.
On the contrary, numerous results support a relative autonomy of the two states of sleep. It is possible to achieve elective suppression of PS by a pontine lesion without abolishing slow sleep, although there is usually only PS in pontine animals (in which it is not possible to observe behavioral or EEG phases of slow sleep), thus demonstrating duality of the structures in action. To this structural duality we may add some arguments in favor of some duality of mechanisms. A unitary theory of sleep necessarily implicates a preceding slow (light) sleep on PS, which is considered a phase of deep sleep According to this hypothesis, there must be some parallelism between the two states of sleep during their phylo- and ontogenetic evolution. Now neither ontogenetic nor phylogenetic data support such an idea. If slow sleep exists in all vertebrates (observed by polygraphic methods from the tortoise to man) PS, on the contrary, does not appear to be connected with the appearance of slow sleep during phylogenetic development. The lack of PS in chelonians and its very rudimentary aspect in birds contrast with its being relatively common and important in mammals. Though the number of animal species studied so far is still too small to draw permanent conclusions, it seems likely that PS occurs from the stage of birds upward during evolution. So we may suppose a new function to appear, not necessarily bound to slow sleep, since it is lacking in reptiles.
The data of ontogenesis also allow us to differentiate the development of the two states of sleep. At birth PS is relatively important and is totally independent of the slow sleep state. So, in newborn mammals, slow sleep is not necessary for the occurrence of PS. Consequently, we must assume the mechanism responsible for PS is pre-established at birth, whereas slow sleep is still little developed and seems to be governed by mechanisms acquired during postnatal maturation.
If, in the adult, the differentiation of behavioral sleep into two states is possible by means of the polygraph, the usual precedence of slow sleep over PS would allow us to assume that slow sleep is necessary for the appearance of PS. However, the selective deprivation technique enables us to dissociate them, since PS may be ob served immediately after wakefulness during recovery. This has also been noticed in adult man during narcoleptic or cataleptic attacks (368).
Slow sleep and PS appear, then, to be the manifestation of two processes, relatively opposite in their structures and mechanisms; thus it would be better to refer to different states rather than phases of sleep. Behavioral sleep in the adult mammal does not seem to develop according to a cycle, from light to deep sleep, but seems to include two states of activity, qualitatively different, of the nervous system. It is possible that these two states of activity are induced by different groups of monoaminergic neurons, as suggested in a possible monoaminergic theory of sleep, which is summarized next.
VII. A possible monoaminergic theory of sleep
Sleep cannot be explained any more by the passive relaxation of the wakeful ness system since transection (30) or limited lesions of the brain stem lead to a very marked increase in arousal. Thus there is little doubt that the problem of sleep is limited to the knowledge of the neural structures and mechanisms that periodically damp down the reticular activating system. This problem is, however, complicated by the fact that two different states of sleep (SWS and PS) are involved successively.
There is little doubt that the neural structures responsible for behavioral sleep are mainly, if not exclusively, located in the lower brain stem, and it appears al most certain that rostral structures (mostly-telencephalic) are necessary for the occurrence of synchronization (but not necessarily sleep) at the cortical and subcortical levels.
The search for sleep-inducing structures is thus limited to the brain stem. Whether these structures belong to some specific nuclei in the medulla or pons or to a discrete group of monoaminergic (serotonergic) neurons located mostly in the raphe system probably shall be solved in the near future.
The essential role played by the pontine tegmentum for the triggering of most tonic and phasic phenomena of PS is also supported by numerous experimental data, and it is very unlikely that any structures situated rostrally to the pons should play a determinant role in the induction of PS since the essential components of PS still appear periodically in chronic pontine cats. It should be emphasized that a group of noradrenergic neurons is apparently located in the dorsal part of the mediolateral pontine reticular formation, which plays a determinant role in the kiggering of PS. Thus both serotonergic neurons of the raphe complex and the catecholaminergic neurons of the pontine tegmentum appear to have a strategic and determinant position in the triggering of both states of sleep. Their intervention in sleep mechanisms is strongly supported by neuropharmacological data in brief, any alteration in brain monoamines leads to specific and obvious alteration of sleep, any increase in brain serotonin leads to an increase in SWS (and a parallel decrease in PS), and blockage of mono-amino-oxidase leads to elective suppression of PS whereas the release of monoamines at the monoaminergic terminals induced by reserpine leads to the elective occurrence of the specific PGO activity of PS.
There are many histochemical (114) and biochemical (193, 194) data that support the existence of an ascending serotonergic system with cells located mainly in the raphe system. The intervention of such an ascending system in the mecha nism of SWS appears likely since its almost total destruction led to a state of almost permanent wakefulness, whereas any attempt to increase the 5-HT content of the serotonergic neurons (which is revealed by the increase of the yellow fluorescence after injection of Nialamide) (114) led to an increase in SWS.
The process by which serotonergic neurons could act on the arousal system during SWS is still unknown and more facts about the amounts of bound and free 5-HT in the brain are required.
If the process governing SWS appears to be related to the entry of 5-HT in serotonergic cell bodies, the process of PS appears to be related to the release of monoamines at the monoaminergic terminals, since reserpine can trigger electively the most specific electrical phasic activity of PS; the existence of catecholaminergic mechanisms is strongly suggested by the histochemical structures of the dorsal part of the mediolateral pontine tegmentum (noradrenergic neurons) [group A6 of Dahlstrom and Fuxe (114)] and by the fact that the total atony of PS may appear if DOPA is injected after reserpine.
The physiological mechanism, which periodically acts (possibly at the mem brane level of monoaminergic terminals during PS) in a very subtle way almost similar to reserpine, is still a matter of speculation Thus, a very discrete sleep "lobby" composed of monoaminergic neurons, with cell bodies mostly located in the raphe system and the pontine tegmentum, appears to influence periodically during SWS and PS all other far more numerous "classical" neurons of the brain. For what purposes, during phylogeny, monoaminergic neurons were first responsible for sleep, and then for both sleep and dreaming, is a great challenge to neurophysiologists.
The author is much indebted to Prof Eric Neil, who undertook the difficult task of correcting the English version of this paper.
Fig. 1 : Polygraphic aspects of the 2 states of sleep
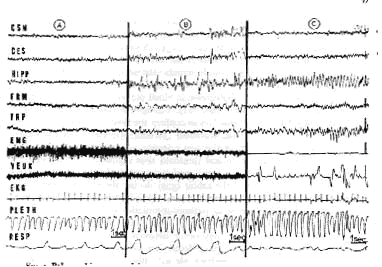
A (wakefulness) fast cortical and subcortical activity; increased EMG activity.
B (slow sleep) cortical spindles and slow waves; high-voltage spikes at ventral hippocampus (HIPP); slow waves in mesencephalic reticular formation (FRM); decrease in EMG activity of the neck.
C (paradoxical sleep) low-voltage fast cortical activity similar to wakefulness; regular theta activity at the ventral hippocampus; phasic activity in pontile reticular formation (FRP); total disappearance of EMG activity of the neck; clusters of rapid eye movements (EYES); change in respiratory activity (RESP) and plethysmographic index of the front leg (PLETH). CSM = sensorimotor cortex; CES = ectosylvian cortex. Scale 1 sec, 50 microv.
From Jouvet (240)
Fig. 2. Phasic EEG activity during paradoxical sleep
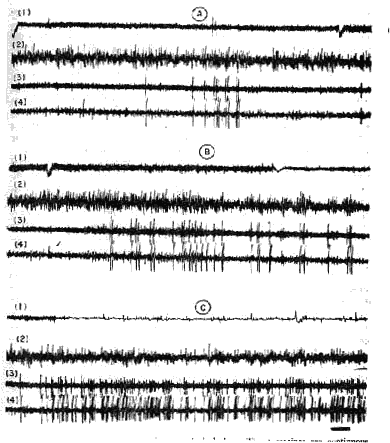
The 3 tracings are continuous (each line represents 2 min).
A slow sleep with slow cortical activity, occipItal. cortex (2), and with isolated PGO activity, monophasic spikes at right (3) and left (4) lateral geniculate bodies.
B beginning of PS PG0 activity occurs permanently 60 sec before disappearance of EMG activity of the neck (1).
C PS increase in the bursts of monophasic spikes whereupon there is a fast cortical activity with occipItal. spikes. Scale 6 sec, 50 microv.